Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects - PubMed (original) (raw)
Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects
Francesco Angelucci et al. J Biol Chem. 2009.
Abstract
Schistosomiasis is a parasitic disease affecting over 200 million people currently treated with one drug, praziquantel. A possible drug target is the seleno-protein thioredoxin-glutathione reductase (TGR), a key enzyme in the pathway of the parasite for detoxification of reactive oxygen species. The enzyme is a unique fusion of a glutaredoxin domain with a thioredoxin reductase domain, which contains a selenocysteine (Sec) as the penultimate amino acid. Auranofin (AF), a gold-containing compound already in clinical use as an anti-arthritic drug, has been shown to inhibit TGR and to substantially reduce worm burden in mice. Using x-ray crystallography we solved (at 2.5 A resolution) the structure of wild type TGR incubated with AF. The electron density maps show that the actual inhibitor is gold, released from AF. Gold is bound at three different sites not directly involving the C-terminal Sec residue; however, because the C terminus in the electron density maps is disordered, we cannot exclude the possibility that gold may also bind to Sec. To investigate the possible role of Sec in the inactivation kinetics, we tested the effect of AF on a model enzyme of the same superfamily, i.e. the naturally Sec-lacking glutathione reductase, and on truncated TGR. We demonstrate that the role of selenium in the onset of inhibition by AF is catalytic and can be mimicked by an external source of selenium (benzeneselenol). Therefore, we propose that Sec mediates the transfer of gold from its ligands in AF to the redox-active Cys couples of TGR.
Figures
SCHEME 1.
Possible reaction path for AF and SmTGR. One molecule of AF reacts with wild type reduced SmTGR (species 1) to yield a transient intermediate bearing an Au(I) coordinated with Sec597 and triethylphosphine (species 2); we hypothesize that acetoxythioglucose is released first (see text). The intermediate species releases triethylphosphine to form a Sec-gold-Cys complex with any of the available cysteinyl residues; three reaction products are possible (species 3, 4, and 6). The chemical species in which the C-terminal arm is cross-linked via the gold ion to a distant Cys residue (species 4 and 6) may rearrange to yield the more stable short distance Cys-gold-Cys complexes (species 5 and 7). When equilibrium is reached, a mixture of species 3, 5, and 7 is expected to be present, dynamically interchanging via the less stable intermediates 4 and 6. Additional molecules of AF may be introduced, leading to SmTGR molecules bearing two or three gold ions. The gold-binding site 3, in which the metal ion is not coordinated by Cys or Sec, is not considered in the scheme.
FIGURE 1.
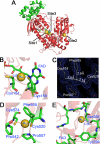
The three gold-binding sites of wild type SmTGR. A, the three-dimensional model of one subunit is shown with the three gold-binding sites. Site 1 shows the gold in between Cys154 and Cys159; site 2 shows the gold in between Cys520 and Cys574; site 3 shows the gold in the putative NADPH-binding pocket. The glutaredoxin domain of TGR is shown in green, whereas the thioredoxin domain is shown in red. The bound flavin is also highlighted. B, site 1. The linear geometry of the Cys154-gold-Cys159 adduct is shown. The occupancy of the gold atom is about 50%. Both distances of the sulfur-gold bond are 2.3 Å, as expected for this type of coordination moiety. C, site 2. The electron density map (2_F_o − _F_c) contoured at 1 σ shows the possible charge transfer complex between the gold and Phe505. D, site 2. The gold atom between Cys574 and Cys520 is shown together with the other residues that surround the metal, i.e. Phe505, Pro507, and Pro542. E, site 3. Gold in the putative NADPH-binding site of SmTGR. Tyr296 is known to swing upon NADPH binding in thiol reductase enzymes (30). Ser295 is the residue closest to the gold (Ser295(OG)-gold: 3.2 Å). Other van der Waals' contacts are with the main chain atoms of the polypeptide (Ala390, Val391, Gly392, and Arg393).
FIGURE 2.
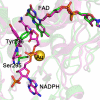
The SmTGR crystal structure in complex with gold ions (in green) is superimposed to the mouse TR with NADPH bound (in magenta; Protein Data Bank code 1zdl (30)). The root mean square deviation is 0.82 Å over the 462 aligned residues. The residues surrounding NADPH in mouse TR are conserved in SmTGR (sequence alignment not shown). The structural comparison shows the change in conformation of the loop 293–296 and in particular of Tyr296 and Ser295, highlighted for the two enzymes as balls and sticks (the other amino acid side chains are omitted for clarity). The OG atom of Ser295 is the closest contact with the gold ion in the SmTGR crystal structure (see “Results” and Fig. 1). In the mouse TR structure, Ser295 shifts in position to make room for the bound NADPH; the clash between the metal in site 3 and the phosphate of the cofactor in SmTGR is self-evident.
FIGURE 3.
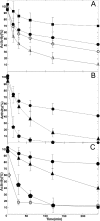
A, baker's yeast GR is inactivated by AF: time courses obtained at concentrations of 1 μ
m
(squares), 4 μ
m
(closed circles), 10 μ
m
(open circles), and 50 μ
m
(open triangles). B, effect of BzSe on baker's yeast GR inactivation by AF. Time course in the presence of 4 μ
m
AF (circles) or 4 μ
m
AF plus 2 μ
m
BzSe (triangles). GR exposed to a mixture in which AF 4 μ
m
and BzSe 2 μ
m
were preincubated for 2 h before the assay (pentagons). C, inactivation by AF of truncated SmTGR and wild type SmTGR, and the effect of BzSe. Time courses of truncated SmTGR in the presence of 8 μ
m
AF (circles) or 8 μ
m
AF plus 3 μ
m
BzSe (triangles). Truncated SmTGR exposed to a mixture in which AF 8 μ
m
and BzSe 3 μ
m
were preincubated for 2 h before the assay (pentagons); time course of wild type SmTGR 20 n
m
incubated with 50 n
m
AF (open circles).
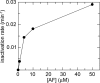
FIGURE 4.
Rate constant of inactivation (min−1) of yeast GR (0.5 μ
m
) as a function of AF concentration (1, 4, 10, and 50 μ
m
), as derived from linearization of the first points of the time courses reported for Fig. 3_A_.
Similar articles
- Thioredoxin glutathione reductase as a novel drug target: evidence from Schistosoma japonicum.
Song L, Li J, Xie S, Qian C, Wang J, Zhang W, Yin X, Hua Z, Yu C. Song L, et al. PLoS One. 2012;7(2):e31456. doi: 10.1371/journal.pone.0031456. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384025 Free PMC article. - Mapping the catalytic cycle of Schistosoma mansoni thioredoxin glutathione reductase by X-ray crystallography.
Angelucci F, Dimastrogiovanni D, Boumis G, Brunori M, Miele AE, Saccoccia F, Bellelli A. Angelucci F, et al. J Biol Chem. 2010 Oct 15;285(42):32557-67. doi: 10.1074/jbc.M110.141960. Epub 2010 Jul 21. J Biol Chem. 2010. PMID: 20659890 Free PMC article. - Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione.
Bonilla M, Denicola A, Novoselov SV, Turanov AA, Protasio A, Izmendi D, Gladyshev VN, Salinas G. Bonilla M, et al. J Biol Chem. 2008 Jun 27;283(26):17898-907. doi: 10.1074/jbc.M710609200. Epub 2008 Apr 11. J Biol Chem. 2008. PMID: 18408002 Free PMC article. - Macromolecular bases of antischistosomal therapy.
Angelucci F, Miele AE, Boumis G, Brunori M, Dimastrogiovanni D, Bellelli A. Angelucci F, et al. Curr Top Med Chem. 2011;11(16):2012-28. doi: 10.2174/156802611796575939. Curr Top Med Chem. 2011. PMID: 21619508 Review. - Targeting thioredoxin glutathione reductase as a potential antischistosomal drug target.
Eweas AF, Allam G. Eweas AF, et al. Mol Biochem Parasitol. 2018 Oct;225:94-102. doi: 10.1016/j.molbiopara.2018.09.004. Epub 2018 Oct 4. Mol Biochem Parasitol. 2018. PMID: 30291946 Review.
Cited by
- A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia.
Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Debnath A, Gut J, McKerrow JH, Reed SL, Eckmann L. Tejman-Yarden N, et al. Antimicrob Agents Chemother. 2013 May;57(5):2029-35. doi: 10.1128/AAC.01675-12. Epub 2013 Feb 12. Antimicrob Agents Chemother. 2013. PMID: 23403423 Free PMC article. - A comprehensive exploration of schistosomiasis: Global impact, molecular characterization, drug discovery, artificial intelligence and future prospects.
Ekloh W, Asafu-Adjaye A, Tawiah-Mensah CNL, Ayivi-Tosuh SM, Quartey NK, Aiduenu AF, Gayi BK, Koudonu JAM, Basing LA, Yamoah JAA, Dofuor AK, Osei JHN. Ekloh W, et al. Heliyon. 2024 Jun 14;10(12):e33070. doi: 10.1016/j.heliyon.2024.e33070. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38988508 Free PMC article. Review. - Characterization of Entamoeba histolytica adenosine 5'-phosphosulfate (APS) kinase; validation as a target and provision of leads for the development of new drugs against amoebiasis.
Mi-Ichi F, Ishikawa T, Tam VK, Deloer S, Hamano S, Hamada T, Yoshida H. Mi-Ichi F, et al. PLoS Negl Trop Dis. 2019 Aug 19;13(8):e0007633. doi: 10.1371/journal.pntd.0007633. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31425516 Free PMC article. - Biscarbene gold(i) complexes: structure-activity-relationships regarding antibacterial effects, cytotoxicity, TrxR inhibition and cellular bioavailability.
Schmidt C, Karge B, Misgeld R, Prokop A, Brönstrup M, Ott I. Schmidt C, et al. Medchemcomm. 2017 Jun 27;8(8):1681-1689. doi: 10.1039/c7md00269f. eCollection 2017 Aug 1. Medchemcomm. 2017. PMID: 30108879 Free PMC article. - Evaluation of Auranofin Loading within Ferritin Nanocages.
Lucignano R, Pratesi A, Imbimbo P, Monti DM, Picone D, Messori L, Ferraro G, Merlino A. Lucignano R, et al. Int J Mol Sci. 2022 Nov 16;23(22):14162. doi: 10.3390/ijms232214162. Int J Mol Sci. 2022. PMID: 36430642 Free PMC article.
References
- Hotez P. J., Molyneux D. H., Fenwick A., Kumaresan J., Sachs S. E., Sachs J. D., Savioli L. (2007) N. Engl. J. Med. 357, 1018–1027 - PubMed
- Doenhoff M. J., Kusel J. R., Coles G. C., Cioli D. (2002) Trans. R. Soc. Trop. Med. Hyg. 96, 465–469 - PubMed
- Angelucci F., Basso A., Bellelli A., Brunori M., Pica-Mattoccia L., Valle C. (2007) Parasitology 134, 1215–1221 - PubMed
- Cioli D., Valle C., Angelucci F., Miele A. E. (2008) Trends Parasitol. 24, 379–382 - PubMed
- Salinas G., Selkirk M. E., Chalar C., Maizels R. M., Fernández C. (2004) Trends Parasitol. 20, 340–346 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources