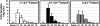The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Oct;90(4):1029-37.
doi: 10.3945/ajcn.2009.27981. Epub 2009 Aug 26.
Affiliations
- PMID: 19710188
- PMCID: PMC2744624
- DOI: 10.3945/ajcn.2009.27981
Randomized Controlled Trial
The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers
Kenneth Dr Setchell et al. Am J Clin Nutr. 2009 Oct.
Abstract
Background: The nonsteroidal estrogen equol occurs as diastereoisomers, S-(-)equol and R-(+)equol, both of which have significant biological actions. S-(-)equol, the naturally occurring enantiomer produced by 20-30% of adults consuming soy foods, has selective affinity for estrogen receptor-beta, whereas both enantiomers modulate androgen action. Little is known about the pharmacokinetics of the diastereoisomers, despite current interest in developing equol as a nutraceutical or pharmaceutical agent.
Objective: The objective was to compare the pharmacokinetics of S-(-)equol and R-(+)equol by using [13C] stable-isotope-labeled tracers to facilitate the optimization of clinical studies aimed at evaluating the potential of these diastereoisomers in the prevention and treatment of estrogen- and androgen-dependent conditions.
Design: A randomized, crossover, open-label study in 12 healthy adults (6 men and 6 women) compared the plasma and urinary pharmacokinetics of orally administered enantiomeric pure forms of S-(-)[2-13C]equol, R-(+)[2-13C]equol, and the racemic mixture. Plasma and urinary [13C]R-equol and [13C]S-equol concentrations were measured by tandem mass spectrometry.
Results: Plasma [13C]equol concentration appearance and disappearance curves showed that both enantiomers were rapidly absorbed, attained high circulating concentrations, and had a similar terminal elimination half-life of 7-8 h. The systemic bioavailability and fractional absorption of R-(+)[2-13C]equol were higher than those of S-(-)[2-13C]equol or the racemate. The pharmacokinetics of racemic (+/-)[2-13C]equol were different from those of the individual enantiomers: slower absorption, lower peak plasma concentrations, and lower systemic bioavailability.
Conclusions: The high bioavailability of both diastereoisomers contrasts with previous findings for the soy isoflavones daidzein and genistein, both of which have relatively poor bioavailability, and suggests that low doses of equol taken twice daily may be sufficient to achieve biological effects.
Figures
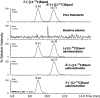
FIGURE 1
Typical chiral phase liquid chromatography tandem mass spectrometry profiles of the negative ion multiple reaction ion monitoring (MRM) transition m/z 242→121 for plasma samples collected 2 h after administration of 20 mg _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol to the same healthy adult. Shown for comparison is a sample of plasma taken at baseline and the same sample after adding the enantiomeric pure standards of _S_-(−)[2-13C]equol and _R_-(+)[2-13C]equol. These analyses established a lack of biotransformation of the individual enantiomers after oral administration.
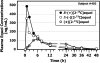
FIGURE 2
Typical plasma equol concentration appearance and disappearance curves obtained in a healthy adult after administration of a single-bolus, 20-mg dose of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol.
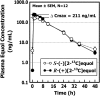
FIGURE 3
Log linear plots of appearance and disappearance curves for mean (±SD) plasma _S_-(−)[2-13C]equol and _R_-(+)[2-13C]equol concentrations obtained in the same 12 healthy adults after administration of a single-bolus, 20-mg dose of _S_-(−)[2-13C]equol and _R_-(+)[2-13C]equol administered in a randomized crossover design study; data for (±)[2-13C]equol are not included so that the comparison of the characteristics of the 2 enantiomers is clear. _C_max, maximum plasma concentration.
FIGURE 4
Cumulative urinary excretion of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol.
FIGURE 5
Mean (±SD) urinary excretion of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol in 12-h pooled collections over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol.
FIGURE 6
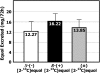
Mean (±SD) total recovery of _S_-(−)[2-13C]equol [_S_-(−)], _R_-(+)[2-13C]equol [_R_-(+)], and (±)[2-13C]equol (±) over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of _S_-(−)[2-13C]equol, _R_-(+)[2-13C]equol, and (±)[2-13C]equol. Urinary recovery of _R_-(+)[2-13C]equol was higher than that of either _S_-(−)[2-13C]equol or (±)[2-13C], but the differences were not statistically significant when calculated by repeated-measures ANOVA.
Similar articles
- S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora.
Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. Setchell KD, et al. Am J Clin Nutr. 2005 May;81(5):1072-9. doi: 10.1093/ajcn/81.5.1072. Am J Clin Nutr. 2005. PMID: 15883431 Clinical Trial. - Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women.
Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A. Setchell KD, et al. Am J Clin Nutr. 2003 Feb;77(2):411-9. doi: 10.1093/ajcn/77.2.411. Am J Clin Nutr. 2003. PMID: 12540402 Clinical Trial. - The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women.
Setchell KD, Zhao X, Shoaf SE, Ragland K. Setchell KD, et al. J Nutr. 2009 Nov;139(11):2037-43. doi: 10.3945/jn.109.110874. Epub 2009 Sep 23. J Nutr. 2009. PMID: 19776178 Clinical Trial. - Equol: history, chemistry, and formation.
Setchell KD, Clerici C. Setchell KD, et al. J Nutr. 2010 Jul;140(7):1355S-62S. doi: 10.3945/jn.109.119776. Epub 2010 Jun 2. J Nutr. 2010. PMID: 20519412 Free PMC article. Review. - Equol: pharmacokinetics and biological actions.
Setchell KD, Clerici C. Setchell KD, et al. J Nutr. 2010 Jul;140(7):1363S-8S. doi: 10.3945/jn.109.119784. Epub 2010 Jun 2. J Nutr. 2010. PMID: 20519411 Free PMC article. Review.
Cited by
- Calcium-41: a technology for monitoring changes in bone mineral.
Weaver CM, Martin BR, Jackson GS, McCabe GP, Peacock M, Wastney M. Weaver CM, et al. Osteoporos Int. 2017 Apr;28(4):1215-1223. doi: 10.1007/s00198-016-3849-3. Epub 2016 Dec 7. Osteoporos Int. 2017. PMID: 27928628 Review. - Endogenous and exogenous equol are antiestrogenic in reproductive tissues of apolipoprotein e-null mice.
Dewi FN, Wood CE, Lampe JW, Hullar MA, Franke AA, Golden DL, Adams MR, Cline JM. Dewi FN, et al. J Nutr. 2012 Oct;142(10):1829-35. doi: 10.3945/jn.112.161711. Epub 2012 Aug 29. J Nutr. 2012. PMID: 22933749 Free PMC article. - Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone.
Tuli HS, Kumar A, Sak K, Aggarwal D, Gupta DS, Kaur G, Vashishth K, Dhama K, Kaur J, Saini AK, Varol M, Capanoglu E, Haque S. Tuli HS, et al. Pharmaceuticals (Basel). 2022 Nov 16;15(11):1418. doi: 10.3390/ph15111418. Pharmaceuticals (Basel). 2022. PMID: 36422548 Free PMC article. Review. - Pharmacokinetics and safety profile of single-dose administration of an estrogen receptor β-selective phytoestrogenic (phytoSERM) formulation in perimenopausal and postmenopausal women.
Hernandez G, Zhao L, Franke AA, Chen YL, Mack WJ, Brinton RD, Schneider LS. Hernandez G, et al. Menopause. 2018 Feb;25(2):191-196. doi: 10.1097/GME.0000000000000984. Menopause. 2018. PMID: 28926513 Free PMC article. - Effects of Smoking on Inflammatory Markers in a Healthy Population as Analyzed via the Gut Microbiota.
Yan S, Ma Z, Jiao M, Wang Y, Li A, Ding S. Yan S, et al. Front Cell Infect Microbiol. 2021 Jul 23;11:633242. doi: 10.3389/fcimb.2021.633242. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34368009 Free PMC article.
References
- Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr 1984;40:569–78 - PubMed
- Setchell KDR, Brown NM, Zimmer-Nechemias L, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 2002;76:447–53 - PubMed
- Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 2001;81:735–47 - PubMed
- Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med 2003;53:607–15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources