Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity - PubMed (original) (raw)
. 2009 Sep;60(9):2772-81.
doi: 10.1002/art.24751.
Michiko Suzuki, Marisa Klein-Gitelman, Murray H Passo, Judyann Olson, Nora G Singer, Kathleen A Haines, Karen Onel, Kathleen O'Neil, Earl D Silverman, Lori Tucker, Jun Ying, Prasad Devarajan, Hermine I Brunner
Affiliations
- PMID: 19714584
- PMCID: PMC3064260
- DOI: 10.1002/art.24751
Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity
Claas H Hinze et al. Arthritis Rheum. 2009 Sep.
Abstract
Objective: To determine whether neutrophil gelatinase-associated lipocalin (NGAL) can predict worsening of global and renal disease activity in childhood-onset systemic lupus erythematosus (SLE).
Methods: One hundred eleven patients with childhood-onset SLE were enrolled in a longitudinal, prospective study with quarterly study visits and had at least 3 study visits. At each visit, global disease activity was measured using 3 external standards: the numerically converted British Isles Lupus Assessment Group (BILAG) index, the SLE Disease Activity Index 2000 update score, and the physician's assessment of global disease activity. Renal and extrarenal disease activity were measured by the respective domain scores. The disease course over time was categorized at the most recent visit (persistently active, persistently inactive, improved, or worsening). Plasma and urinary NGAL levels were measured by enzyme-linked immunosorbent assay, and urinary NGAL levels were standardized to the urinary creatinine concentration. The longitudinal changes in NGAL levels were compared with the changes in SLE disease activity using mixed-effect models.
Results: Significant increases in standardized urinary NGAL levels of up to 104% were detected up to 3 months before worsening of lupus nephritis (as measured by all 3 external standards). Plasma NGAL levels increased significantly by as much as 26% up to 3 months before worsening of global SLE disease activity as measured by all 3 external standards. Plasma NGAL levels increased significantly by 26% as early as 3 months prior to worsening of lupus nephritis as measured by the BILAG renal score.
Conclusion: Serial measurement of urinary and plasma NGAL levels may be valuable in predicting impending worsening of global and renal childhood-onset SLE disease activity.
Figures
Figure 1
Categorization of the Disease Course with childhood-onset SLE (A) The disease course was categorized as persistently active, persistently inactive, improved or worsening. For patients to be categorized as having persistently active (inactive) disease the disease activity score had to remain above (below) a predefined threshold and the change could not exceed a predefined magnitude. If the change exceeded a predefined magnitude, patients were categorized as having improved (if decreased score) or worsening (if increased score) disease activity. (B) The predefined thresholds and required changes are shown. * MD-Global: Physician global assessment score measured on a 10 cm visual analog scale; 0 = inactive SLE. ** MD-Renal: Physician renal assessment score measured on a 10 cm visual analog scale; 0 = inactive SLE renal disease. † SLEDAI-2K-Global/Extrarenal: Systemic Lupus Erythematosus Disease Activity Index 2000 Update global/extrarenal score; range 0 – 105/0–89; 0 = inactive SLE § SLEDAI-2K-Renal: Systemic Lupus Erythematosus Disease Activity Index 2000 Update renal score; range 0 – 16; 0 = inactive SLE renal disease. ‡ BILAG-Global/Extrarenal: British Isle Lupus Assessment Group global/extrarenal score; range 0–72/0–63; 0 = inactive SLE. ¶ BILAG-Renal: British Isle Lupus Assessment Group renal score; range 0–9; 0 = inactive SLE renal disease.
Figure 2
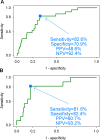
ROC curves of predicting worsening of renal disease activity. The sensitivity, specificity of the predicted probability of worsening, estimated from multivariate logistic regression, are represented, using (A) the SLEDAI-2K-Renal as the external standard (AUC = 0.78) “and (B) the BILAG-Renal as the external standard (AUC = 0.80).
Similar articles
- Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus.
Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P. Brunner HI, et al. Arthritis Rheum. 2006 Aug;54(8):2577-84. doi: 10.1002/art.22008. Arthritis Rheum. 2006. PMID: 16868980 - Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis.
Torres-Salido MT, Cortés-Hernández J, Vidal X, Pedrosa A, Vilardell-Tarrés M, Ordi-Ros J. Torres-Salido MT, et al. Nephrol Dial Transplant. 2014 Sep;29(9):1740-9. doi: 10.1093/ndt/gfu062. Epub 2014 Apr 7. Nephrol Dial Transplant. 2014. PMID: 24711435 - Urinary neutrophil gelatinase-associated lipocalin as a marker of severe lupus nephritis in children.
Hammad A, Mosaad Y, Elhanbly S, Youssef H, El Refaaey A, Elhusseini F, Bakr A. Hammad A, et al. Lupus. 2013 Apr;22(5):486-91. doi: 10.1177/0961203313479419. Lupus. 2013. PMID: 23554037 - The novel role of neutrophil gelatinase-B associated lipocalin (NGAL)/Lipocalin-2 as a biomarker for lupus nephritis.
Rubinstein T, Pitashny M, Putterman C. Rubinstein T, et al. Autoimmun Rev. 2008 Jan;7(3):229-34. doi: 10.1016/j.autrev.2007.11.013. Epub 2007 Dec 3. Autoimmun Rev. 2008. PMID: 18190883 Review. - Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients.
Hjortrup PB, Haase N, Wetterslev M, Perner A. Hjortrup PB, et al. Crit Care. 2013 Apr 24;17(2):211. doi: 10.1186/cc11855. Crit Care. 2013. PMID: 23680259 Free PMC article. Review.
Cited by
- Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus.
Rullo OJ, Woo JM, Parsa MF, Hoftman AD, Maranian P, Elashoff DA, Niewold TB, Grossman JM, Hahn BH, McMahon M, McCurdy DK, Tsao BP. Rullo OJ, et al. Arthritis Res Ther. 2013 Jan 23;15(1):R18. doi: 10.1186/ar4150. Arthritis Res Ther. 2013. PMID: 23343383 Free PMC article. - Metabolic Factors that Contribute to Lupus Pathogenesis.
Li W, Sivakumar R, Titov AA, Choi SC, Morel L. Li W, et al. Crit Rev Immunol. 2016;36(1):75-98. doi: 10.1615/CritRevImmunol.2016017164. Crit Rev Immunol. 2016. PMID: 27480903 Free PMC article. Review. - Proteomic profiling of urine: implications for lupus nephritis.
Aljaberi N, Bennett M, Brunner HI, Devarajan P. Aljaberi N, et al. Expert Rev Proteomics. 2019 Apr;16(4):303-313. doi: 10.1080/14789450.2019.1592681. Epub 2019 Mar 18. Expert Rev Proteomics. 2019. PMID: 30855196 Free PMC article. Review. - Pediatric SLE--towards a comprehensive management plan.
Brunner HI, Huggins J, Klein-Gitelman MS. Brunner HI, et al. Nat Rev Rheumatol. 2011 Apr;7(4):225-33. doi: 10.1038/nrrheum.2011.15. Epub 2011 Mar 8. Nat Rev Rheumatol. 2011. PMID: 21386795 Review. - Neutrophil gelatinase-associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis in mice.
Pawar RD, Pitashny M, Gindea S, Tieng AT, Levine B, Goilav B, Campbell SR, Xia Y, Qing X, Thomas DB, Herlitz L, Berger T, Mak TW, Putterman C. Pawar RD, et al. Arthritis Rheum. 2012 May;64(5):1620-31. doi: 10.1002/art.33485. Arthritis Rheum. 2012. PMID: 22083497 Free PMC article.
References
- Hoffman IE, Lauwerys BR, de Keyser F, Huizinga TW, Isenberg DA, Cebecauer L, et al. Juvenile onset systemic lupus erythematosus: different clinical and serological pattern compared to adult onset systemic lupus erythematosus. Ann Rheum Dis. 2008 - PubMed
- Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58(2):556–62. - PubMed
- Bogdanovic R, Nikolic V, Pasic S, Dimitrijevic J, Lipkovska-Markovic J, Eric-Marinkovic J, et al. Lupus nephritis in childhood: a review of 53 patients followed at a single center. Pediatr Nephrol. 2004;19(1):36–44. - PubMed
- Hagelberg S, Lee Y, Bargman J, Mah G, Schneider R, Laskin C, et al. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002;29(12):2635–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK 069749/DK/NIDDK NIH HHS/United States
- P50 DK 52612/DK/NIDDK NIH HHS/United States
- R01 DK069749/DK/NIDDK NIH HHS/United States
- R01 DK 53289/DK/NIDDK NIH HHS/United States
- P60 AR047784/AR/NIAMS NIH HHS/United States
- R21 DK 070163/DK/NIDDK NIH HHS/United States
- P60 AR 47784/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous