Eosinophil ribonucleases and their cutaneous lesion-forming activity - PubMed (original) (raw)
Eosinophil ribonucleases and their cutaneous lesion-forming activity
Douglas A Plager et al. J Immunol. 2009.
Abstract
Eosinophil granule proteins are deposited in cutaneous lesions in many human diseases, but how these proteins contribute to pathophysiology is obscure. We injected eosinophil cationic protein (ECP or RNase 3), eosinophil-derived neurotoxin (EDN or RNase 2), eosinophil peroxidase (EPO), and major basic protein-1 (MBP1) intradermally into guinea pig and rabbit skin. ECP and EDN each induced distinct skin lesions at >or=2.5 microM that began at 2 days, peaking at approximately 7 days and persisting up to 6 wk. These lesions were ulcerated (ECP) or crusted (EDN) with marked cellular infiltration. EPO and MBP1 (10 microM) each produced perceptible induration and erythema with moderate cellular infiltration resolving within 2 wk. ECP and EDN localized to dermal cells within 2 days, whereas EPO and MBP1 remained extracellular. Overall, cellular localization and RNase activity of ECP and EDN were critical for lesion formation; differential glycosylation, net cationic charge, or RNase activity alone did not account for lesion formation. Ulcerated lesions from patients with the hypereosinophilic syndrome showed ECP and EDN deposition comparable to that in guinea pig skin. In conclusion, ECP and EDN disrupt skin integrity and cause inflammation. Their presence in ulcerative skin lesions may explain certain findings in human eosinophil-associated diseases.
Figures
Figure 1
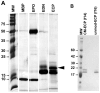
Purity of Eosinophil Granule Proteins. A. An excess of each of the four eosinophil granule proteins was assessed using SDS-PAGE under reducing conditions. EPO is composed of both heavy (~50 kDa) and light (~10 kDa) chains, and the arrowhead points to the most prominent, more highly glycosylated forms of EDN and ECP in their respective lanes (14). B. Carboxymethylated-ECP (CM-ECP) and unmodified-ECP (unmod-ECP) after elution from a second heparin-Sepharose CL-6B column was analyzed by SDS-PAGE under reducing conditions. Note that a greater proportion of the lower ECP-2 band is likely present in the unmod-ECP lane because the peak of unmodified-ECP, which was from exactly the same ECP sample as that used to generate the CM-ECP, eluted across two main fractions, and it was the latter of these fractions (#16; i.e. f16) that was used for this SDS-PAGE.
Figure 2
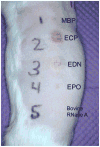
Cutaneous Lesions Induced by Eosinophil Granule Proteins in Guinea Pig Skin. Fifty microliters of proteins, each at 10 μM, were injected intradermally. Proteins were injected to the right of the numeric labels and included MBP1 (1), ECP (2), EDN (3), EPO (4), and bovine RNase A (5). Seven days after injections, the guinea pig was anesthetized and photographed as shown.
Figure 3
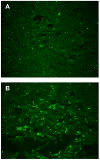
Immunofluorescence Localization of ECP and EDN Following Intradermal Injection. Hematoxylin and eosin staining of the epidermis and upper dermis (A) and the subdermal area surrounding the trunci muscle (B) three days after injection of 10 μM ECP (magnification × 200) shows prominent cellular infiltration in the dermis. Immunofluorescence staining for EDN in the dermis less than one hour (C), two days (D), and four days (E) after intradermal injection at 10 μM (magnification × 400) shows generally diffuse deposition on cells and tissues (C) at one hour whereas the later injection sites (D, E) show an increased proportion of cell-localized staining. Immunofluorescence staining for ECP (F), EPO (G), and MBP1 (H) four days after intradermal injection at 10 μM (magnification × 400) shows relatively greater cellular staining for ECP, similar to EDN, compared to the predominantly extracellular staining for EPO and MBP1.
Figure 4
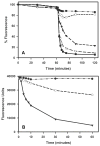
RNase Activity of Unmodified and Carboxymethylated RNases. A. RNase activity, reflected by a decrease in fluorescence, after 60 min equilibration, is shown for PBS (closed circles), Ang (open circles), ECP (closed triangles), EDN (open triangles), and bovine RNase A (closed squares). Each protein was added at 1 μg, and data are plotted as the percent of maximum fluorescence. B. An equal volume of the Heparin Sepharose CL-6B column buffer (closed circles), the peak fraction of CM-EDN (absorbance at 280 nm = 0.205; open circles), and the peak fraction of unmodified-EDN (absorbance at 280 nm = 0.271; closed triangles) were each added to separate wells in duplicate after the 60 min equilibration. Initial RNase velocities (the change in fluorescence over the two minute interval beginning two minutes after protein addition and normalized per unit of absorbance at 280 nm) for CM-EDN (911 / 2 / 0.205 = 2,222) and for unmodified-EDN (9121 / 2 / 0.275 = 16,584) indicated an 87% reduction of RNase activity following carboxymethylation of EDN.
Figure 5
Immunofluorescence Staining for Intradermally Injected unmodified-ECP and CM-ECP. Injection site biopsies were taken from one of three guinea pigs three days after injecting unmodified-ECP (A) and CM-ECP (B). Polyclonal rabbit anti-ECP was used to stain for ECP in these specimens, and a representative area of the dermis in each shows comparable cellular and extracellular staining (magnification × 160).
Figure 6
Immunohistologic Staining Similarities to Ulcerated Human Tissue in Hypereosinophilic Syndrome (HES) Lesion. A. Lesion on guinea pig flank skin from ECP injection shows similar appearance to eroded and ulcerated lesions in an HES patient. B. Ulcerations and erosions in patient with HES. C. Oral lesions in HES with central epithelial ulceration and surrounding erythema from which a biopsy specimen was obtained and stained for eosinophil granule proteins. Photomicrographs (magnifications × 160) of serial sections of lesional mucosa (C) from a patient with HES stained with anti-ECP (D), anti-MBP1 (E), anti-EDN (F), and hematoxylin and eosin (G).
Similar articles
- Interactions of eosinophil granule proteins with skin: limits of detection, persistence, and vasopermeabilization.
Davis MD, Plager DA, George TJ, Weiss EA, Gleich GJ, Leiferman KM. Davis MD, et al. J Allergy Clin Immunol. 2003 Nov;112(5):988-94. doi: 10.1016/j.jaci.2003.08.028. J Allergy Clin Immunol. 2003. PMID: 14610493 - Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein.
Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Slifman NR, et al. J Immunol. 1986 Nov 1;137(9):2913-7. J Immunol. 1986. PMID: 3760576 - In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins.
Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. Hamann KJ, et al. J Immunol. 1990 Apr 15;144(8):3166-73. J Immunol. 1990. PMID: 2324497 - The molecular biology of eosinophil granule proteins.
Hamann KJ, Barker RL, Ten RM, Gleich GJ. Hamann KJ, et al. Int Arch Allergy Appl Immunol. 1991;94(1-4):202-9. doi: 10.1159/000235362. Int Arch Allergy Appl Immunol. 1991. PMID: 1657792 Review. - [Biochemistry and biological activities of eosinophil granule proteins].
Enokihara H, Koike T. Enokihara H, et al. Nihon Rinsho. 1993 Mar;51(3):605-12. Nihon Rinsho. 1993. PMID: 7684096 Review. Japanese.
Cited by
- The Intersection of IgE Autoantibodies and Eosinophilia in the Pathogenesis of Bullous Pemphigoid.
Messingham KN, Crowe TP, Fairley JA. Messingham KN, et al. Front Immunol. 2019 Oct 4;10:2331. doi: 10.3389/fimmu.2019.02331. eCollection 2019. Front Immunol. 2019. PMID: 31636640 Free PMC article. Review. - Effects of an Oral CRTh2 Antagonist (AZD1981) on Eosinophil Activity and Symptoms in Chronic Spontaneous Urticaria.
Oliver ET, Chichester K, Devine K, Sterba PM, Wegner C, Vonakis BM, Saini SS. Oliver ET, et al. Int Arch Allergy Immunol. 2019;179(1):21-30. doi: 10.1159/000496162. Epub 2019 Mar 15. Int Arch Allergy Immunol. 2019. PMID: 30879003 Free PMC article. Clinical Trial. - Immune Modulation by Human Secreted RNases at the Extracellular Space.
Lu L, Li J, Moussaoui M, Boix E. Lu L, et al. Front Immunol. 2018 May 16;9:1012. doi: 10.3389/fimmu.2018.01012. eCollection 2018. Front Immunol. 2018. PMID: 29867984 Free PMC article. Review. - Eosinophil Cationic Protein (ECP), a predictive marker of bullous pemphigoid severity and outcome.
Giusti D, Gatouillat G, Le Jan S, Plée J, Bernard P, Antonicelli F, Pham BN. Giusti D, et al. Sci Rep. 2017 Jul 6;7(1):4833. doi: 10.1038/s41598-017-04687-5. Sci Rep. 2017. PMID: 28684769 Free PMC article. - Granulocyte Macrophage Colony-Stimulating Factor-Activated Eosinophils Promote Interleukin-23 Driven Chronic Colitis.
Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F, Schulthess J, McKenzie BS, Crocker PR, Powrie F. Griseri T, et al. Immunity. 2015 Jul 21;43(1):187-99. doi: 10.1016/j.immuni.2015.07.008. Immunity. 2015. PMID: 26200014 Free PMC article.
References
- Kita H, Adolphson CR, Gleich GJ. Biology of Eosinophils. In: Adkinson NF Jr, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergy: Principles and Practice. 6. Mosby; Philadelphia: 2003. pp. 305–332.
- Plager DA, Loegering DA, Weiler DA, Checkel JL, Wagner JM, Clarke NJ, Naylor S, Page SM, Thomas LL, Akerblom I, Cocks B, Stuart S, Gleich GJ. A novel and highly divergent homolog of human eosinophil granule major basic protein. J Biol Chem. 1999;274:14464–14473. - PubMed
- Abu-Ghazaleh RI, Dunnette SL, Loegering DA, Checkel JL, Kita H, Thomas LL, Gleich GJ. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52:611–618. - PubMed
- Leiferman KMPM. Eosinophils in Cutaneous Diseases. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7. McGraw-Hill Medical; New York: 2006. pp. 307–317.
- Davis MD, Plager DA, George TJ, Weiss EA, Gleich GJ, Leiferman KM. Interactions of eosinophil granule proteins with skin: limits of detection, persistence, and vasopermeabilization. J Allergy Clin Immunol. 2003;112:988–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 07047/AI/NIAID NIH HHS/United States
- AI 11483/AI/NIAID NIH HHS/United States
- R01 AI009728/AI/NIAID NIH HHS/United States
- U19 AI034577-08/AI/NIAID NIH HHS/United States
- T32 AI007047-22/AI/NIAID NIH HHS/United States
- AI 34577/AI/NIAID NIH HHS/United States
- P01 AI050494/AI/NIAID NIH HHS/United States
- T32 AI007047/AI/NIAID NIH HHS/United States
- P01 AI050494-029001/AI/NIAID NIH HHS/United States
- AI 50494/AI/NIAID NIH HHS/United States
- AI 09728/AI/NIAID NIH HHS/United States
- R01 AI009728-37/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials