mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse - PubMed (original) (raw)
Review
mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse
Meriem Gaval-Cruz et al. Mol Interv. 2009 Aug.
Abstract
The anti-alcoholism drug disulfiram (Antabuse), which is an inhibitor of aldehyde dehydrogenase, induces an aversive reaction to alcohol consumption and thereby helps patients reduce alcohol intake. Recent clinical trials, initiated to investigate whether disulfiram could be used to treat individuals who abuse both alcohol and cocaine, have indicated that disulfiram effectively decreases cocaine consumption. Yet the ability of disulfiram to curb cocaine intake cannot be explained by the disruption of ethanol metabolism. Here, we synthesize clinical and animal data that point to dopamine beta-hydroxylase inhibition as a mechanism underlying the efficacy of disulfiram in the treatment of cocaine dependence.
Figures
Meriem Gaval-Cruz, BS, is a graduate student in the Division of Biological and Biomedical Sciences at Emory University. Her research interests focus on the neurobiology of drug addiction, with an emphasis on cocaine pharmacotherapies. Her activities include neuroscience outreach to K-12 students and the laboratory mentoring of undergraduates.
David Weinshenker, PhD, is Associate Professor in the Department of Human Genetics at Emory University. He has pursued model systems to better understand genes involved in human disease. He applies genetic models combined with pharmacological tools to investigate various aspects of neurobiology, with a particular focus on the role of norepinephrine in brain function and disease. Send correspondence to DW. E-mail
dweinshenker@genetics.emory.edu
; fax 404-727-3949.
Figure 1
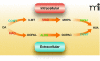
Disulfiram inhibition of ethanol metabolism. Ethanol is first converted into acetaldehyde by alcohol dehydrogenase (ADH). Acetaldehyde is then transformed into acetate by aldehyde dehydrogenase (ALDH). Disulfiram inhibits ALDH and thereby results in "the disulfiram-ethanol reaction" that promotes abstinence from alcohol. See text for details.
Figure 2
Dopamine metabolism. Dopamine (DA) is metabolized intracellularly and extracellularly by the same group of enzymes, but in different orders. Inside dopaminergic cells, monoamine oxidase (MAO) converts dopamine into 3,4-dihydrophenylacetaldeyde (DOPAL), a substrate of aldehyde dehydrogenase (ALDH). ALDH then converts DOPAL into 3,4-dihydroxyphenylacetic acid (DOPAC). After DOPAC diffuses out of the cell, catechol-O-methyltransferase (COMT) converts it into homovanillic acid (HVA). Extracellularly, dopamine metabolism begins by transformation into 3-methoxytyramine (3-MT) by COMT. 3-MT is then oxidized into 3-methoxy-4-hydroxyphenylacetaldehyde (MHPA) by MAO, and finally transformed into HVA by ALDH.
Figure 3
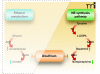
Disulfiram inhibition of the norepinephrine (NE) biosynthetic pathway. In the catecholamine synthesis pathway, tyrosine is converted into 3,4-dihydroxy-L-phenylalanine (L-DOPA) by tyrosine hydroxylase (TH), which is then transformed into dopamine by aromatic amino acid decarboxylase (AADC), whereupon dopamine b-hydroxylase (DBH) converts dopamine into norepinephrine. Disulfiram inhibits DBH, reducing the production of norepinephrine and increasing the pool of dopamine.
Figure 4
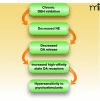
Effect of chronic DBH inhibition on dopamine transmission. Genetic DBH inhibition, and presumably pharmacological DBH inhibition by disulfiram, leads to decreased norepinephrine synthesis in the locus coeruleus and brainstem and norepinephrine release in the midbrain. Because midbrain dopaminergic neurons require noradrenergic drive for normal burst firing and neurotransmitter release, dopamine release is decreased and a compensatory upregulation of high-affinity state dopamine receptors ensues, resulting in behavioral hypersensitivity to psychostimulants.
Similar articles
- Disulfiram: an old therapeutic with new applications.
Barth KS, Malcolm RJ. Barth KS, et al. CNS Neurol Disord Drug Targets. 2010 Mar;9(1):5-12. doi: 10.2174/187152710790966678. CNS Neurol Disord Drug Targets. 2010. PMID: 20201810 Review. - [Disulfiram as a treatment for cocaine dependency].
De Mulder I, Dom G. De Mulder I, et al. Tijdschr Psychiatr. 2012;54(1):51-8. Tijdschr Psychiatr. 2012. PMID: 22237610 Review. Dutch. - Disulfiram: old addiction drug gains new support.
Stezhka T. Stezhka T. Expert Rev Clin Pharmacol. 2013 Mar;6(2):101. Expert Rev Clin Pharmacol. 2013. PMID: 23593718 No abstract available. - Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine β-hydroxylase.
Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Schroeder JP, et al. Neuropsychopharmacology. 2010 Nov;35(12):2440-9. doi: 10.1038/npp.2010.127. Epub 2010 Aug 25. Neuropsychopharmacology. 2010. PMID: 20736996 Free PMC article. - Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine β-hydroxylase.
Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Kosten TR, et al. Biol Psychiatry. 2013 Feb 1;73(3):219-24. doi: 10.1016/j.biopsych.2012.07.011. Epub 2012 Aug 18. Biol Psychiatry. 2013. PMID: 22906516 Free PMC article. Clinical Trial.
Cited by
- Daidzein modulates cocaine-reinforcing effects and cue-induced cocaine reinstatement in CD-1 male mice.
Martin M, Gutiérrez-Martos M, Cabrera R, Langohr K, Maldonado R, Farre M, de la Torre R. Martin M, et al. Psychopharmacology (Berl). 2021 Jul;238(7):1923-1936. doi: 10.1007/s00213-021-05820-z. Epub 2021 Apr 11. Psychopharmacology (Berl). 2021. PMID: 33839903 Free PMC article. - Chronic inhibition of dopamine β-hydroxylase facilitates behavioral responses to cocaine in mice.
Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. Gaval-Cruz M, et al. PLoS One. 2012;7(11):e50583. doi: 10.1371/journal.pone.0050583. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209785 Free PMC article. - A mechanistic overview of approaches for the treatment of psychostimulant dependence.
Jensen KL, Jensen SB, Madsen KL. Jensen KL, et al. Front Pharmacol. 2022 Sep 8;13:854176. doi: 10.3389/fphar.2022.854176. eCollection 2022. Front Pharmacol. 2022. PMID: 36160447 Free PMC article. Review. - Pharmacogenetic role of dopamine transporter (SLC6A3) variation on response to disulfiram treatment for cocaine addiction.
Kampangkaew JP, Spellicy CJ, Nielsen EM, Harding MJ, Ye A, Hamon SC, Kosten TR, Nielsen DA. Kampangkaew JP, et al. Am J Addict. 2019 Jul;28(4):311-317. doi: 10.1111/ajad.12891. Epub 2019 May 14. Am J Addict. 2019. PMID: 31087723 Free PMC article. Clinical Trial. - Disulfiram is a direct and potent inhibitor of human O6-methylguanine-DNA methyltransferase (MGMT) in brain tumor cells and mouse brain and markedly increases the alkylating DNA damage.
Paranjpe A, Zhang R, Ali-Osman F, Bobustuc GC, Srivenugopal KS. Paranjpe A, et al. Carcinogenesis. 2014 Mar;35(3):692-702. doi: 10.1093/carcin/bgt366. Epub 2013 Nov 5. Carcinogenesis. 2014. PMID: 24193513 Free PMC article.
References
- Kitson TM. The disulfiram–ethanol reaction. J Stud Alcohol 38 96–113 (1977). - PubMed
- George TP, Chawarski, MC, Pakes, J, Carroll, KM, Kosten, TR and Schottenfeld, RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: A preliminary trial. Biol Psychiatry 47 1080–1086 (2000). - PubMed
- Petrakis IL, Carroll, KM, Nich, C, Gordon, LT, McCance-Katz, EF, Frankforter, T and Rounsaville, BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95 219–228 (2000). - PubMed
- Carroll KM, Fenton, LR, Ball, SA, Nich, C, Frankforter, TL, Shi, J and Rounsaville, BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized placebo-controlled trial. Arch Gen Psychiatry 61 264–272 (2004). This paper reports the first systematic study of the effect of disulfiram on cocaine use in a general population of cocaine users, regardless of concurrent ethanol intake. It put forth the notion that disulfiram’s mechanism of action in curbing cocaine intake must be independent of its effects on alcohol metabolism. - PMC - PubMed
- Levin EY, Levenberg, B and Kaufman, S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem 235 2080–2086 (1960). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases