Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes - PubMed (original) (raw)
. 2009 Dec;58(12):2893-903.
doi: 10.2337/db09-0320. Epub 2009 Aug 31.
Affiliations
- PMID: 19720799
- PMCID: PMC2780874
- DOI: 10.2337/db09-0320
Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes
Beatriz Duran-Jimenez et al. Diabetes. 2009 Dec.
Abstract
Objective: The goal of this study was to characterize glycation adducts formed in both in vivo extracellular matrix (ECM) proteins of endoneurium from streptozotocin (STZ)-induced diabetic rats and in vitro by glycation of laminin and fibronectin with methylglyoxal and glucose. We also investigated the impact of advanced glycation end product (AGE) residue content of ECM on neurite outgrowth from sensory neurons.
Research design and methods: Glycation, oxidation, and nitration adducts of ECM proteins extracted from the endoneurium of control and STZ-induced diabetic rat sciatic nerve (3-24 weeks post-STZ) and of laminin and fibronectin that had been glycated using glucose or methylglyoxal were examined by liquid chromatography with tandem mass spectrometry. Methylglyoxal-glycated or unmodified ECM proteins were used as substrata for dissociated rat sensory neurons as in vitro models of regeneration.
Results: STZ-induced diabetes produced a significant increase in early glycation N(epsilon)-fructosyl-lysine and AGE residue contents of endoneurial ECM. Glycation of laminin and fibronectin by methylglyoxal and glucose increased glycation adduct residue contents with methylglyoxal-derived hydroimidazolone and N(epsilon)-fructosyl-lysine, respectively, of greatest quantitative importance. Glycation of laminin caused a significant decrease in both neurotrophin-stimulated and preconditioned sensory neurite outgrowth. This decrease was prevented by aminoguanidine. Glycation of fibronectin also decreased preconditioned neurite outgrowth, which was prevented by aminoguanidine and nerve growth factor.
Conclusions: Early glycation and AGE residue content of endoneurial ECM proteins increase markedly in STZ-induced diabetes. Glycation of laminin and fibronectin causes a reduction in neurotrophin-stimulated neurite outgrowth and preconditioned neurite outgrowth. This may provide a mechanism for the failure of collateral sprouting and axonal regeneration in diabetic neuropathy.
Figures
FIG. 1.
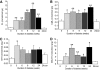
Glycation adduct content of endoneurial ECM proteins of STZ-induced diabetic rat sciatic nerve. Nε-fructosyl-lysine (A), CML (B), G-H1 (C), and MG-H1 (D). Data are given for nondiabetic control (con) rats at 0 and 24 weeks of the experiment and for STZ-induced diabetic rats with duration of diabetes 3–24 weeks (post–STZ injection). Data are means ± SE (n = 6–8 for STZ-induced diabetic groups; n = 4 for nondiabetic controls). Significance: *P < 0.05, **P < 0.01 compared with nondiabetic controls (Kruskal-Wallis). FL, fructosyl-lysine.
FIG. 2.
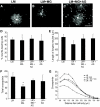
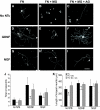
Sensory neurons plated on glycated laminin and treated with NGF extend fewer, less-branched neurites than those plated on control unmodified laminin. Representative photomicrographs of sensory neurons plated on laminin (A), laminin glycated with methylglyoxal (B), or laminin treated with methylglyoxal in the presence of aminoguanidine (C). NGF treatment (10 ng/ml; 18 h) stimulated neurons plated on laminin to extend elaborate highly branched neurites (A). In contrast, NGF-stimulated neurite outgrowth on methylglyoxal-glycated laminin was much less extensive (B); this was prevented by inclusion of aminoguanidine (C). Quantification of NGF-stimulated neurite outgrowth showed no significant reduction in length of longest neurite (E) but a significant reduction in total neurite density and branching structure, compared with control, as measured by cross-point analysis (F and G), which was prevented by inclusion of the glycation scavenger aminoguanidine (C, F, and G). Data are expressed as means ± SD; n = 4 independent cultures (ANOVA and Bonferroni multiple-comparison post hoc test, *P < 0.05). Aminoguanidine treatment alone had no significant effect on any of the indexes examined (_D–G_; _P_ > 0.05). Scale bar = 100 μm. AG, aminoguanidine; LM, laminin; MG, methylglyoxal.
FIG. 3.
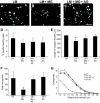
GDNF-induced neurite outgrowth is reduced from sensory neurons plated on glycated laminin. Representative photomicrographs of sensory neurons plated on laminin (A), laminin glycated with methylglyoxal (B), or laminin treated with methylglyoxal in the presence of aminoguanidine (C). GDNF treatment (50 ng/ml; 18 h) stimulated neurons plated on laminin to extend elaborate highly branched neurites (A). In contrast, GDNF-stimulated neurite outgrowth on methylglyoxal-glycated laminin was much less extensive (B); this was prevented by inclusion of aminoguanidine (C). Quantification of GDNF-stimulated neurite outgrowth showed a significant reduction in length of longest neurite (D), total neurite density, and branching structure compared with control, as measured by cross-point analysis (F and G), which was prevented by inclusion of the glycation scavenger aminoguanidine (C, F, and G). Data are expressed as means ± SD, n = 4 independent cultures (ANOVA and Bonferroni multiple-comparison post hoc test, *P < 0.05). Aminoguanidine treatment alone had no significant effect on any of the indexes examined (_D–G_; _P_ > 0.05). Scale bar = 100 μm. AG, aminoguanidine; LM, laminin; MG, methylglyoxal.
FIG. 4.
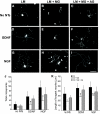
Enhanced neurite outgrowth in response to a preconditioning sciatic nerve crush is reduced in neurons plated on glycated laminin. Representative photomicrographs of neurons from L4 and L5 DRG ipsilateral to a crush injury to the sciatic nerve (7 days before culture), which were dissociated and plated on laminin (A, D, and G), laminin glycated with methylglyoxal (B, E, and H), or laminin treated with methylglyoxal in the presence of aminoguanidine (C, F, and I) for 18 h with (D–I) or without (A–C) neurotrophin treatment (D–F, 50 ng/ml GDNF; G–I, 10 ng/ml NGF). The preconditioned neurons extend neurites in the absence of neurotrophic support (A–C); this was enhanced by neurotrophin treatment (D–I). Two parameters of neurite extension were quantified: total neurite outgrowth (J, cross points) and length of longest neurite (K). Neurons plated on glycated laminin showed a significant reduction in length of longest neurite and total neurite density compared with control, which was prevented by inclusion of the glycation scavenger aminoguanidine (C, F, and I–K). All data are expressed as means ± SD, n = 4 independent experiments (ANOVA with Bonferroni post hoc test, *P < 0.05, **P < 0.01). Scale bar = 100 μm. AG, aminoguanidine; LM, laminin; MG, methylglyoxal.
FIG. 5.
Enhanced neurite outgrowth in response to a preconditioning sciatic nerve crush is reduced in neurons plated on glycated fibronectin. Representative photomicrographs of neurons from L4 and L5 DRG ipsilateral to a crush injury to the sciatic nerve (7 days before culture), which were dissociated and plated on fibronectin (A, D, and G), fibronectin glycated with methylglyoxal (B, E, and H), or fibronectin treated with methylglyoxal in the presence of aminoguanidine (C, F, and I) for 18 h with (D–I) or without (A–C) neurotrophin treatment (D–F, 50 ng/ml GDNF; G–I, 10 ng/ml NGF). The preconditioned neurons extend neurites in the absence of neurotrophic support (A–C); this was enhanced by neurotrophin treatment (D–I). Two parameters of neurite extension were quantified: total neurite outgrowth (J, cross points) and length of longest neurite (K). Neurons plated on glycated fibronectin showed a significant reduction in length of longest neurite and total neurite density compared with control, which was prevented by inclusion of the glycation scavenger aminoguanidine (C, F, and I–K) and also by NGF (H, J, and K). All data are expressed as means ± SD, n = 4 independent experiments (ANOVA with Bonferroni post hoc test, *P < 0.05). Scale bar = 100 μm. AG, aminoguanidine; FN, fibronectin; MG, methylglyoxal.
Similar articles
- Matrix metalloproteinase-2 is downregulated in sciatic nerve by streptozotocin induced diabetes and/or treatment with minocycline: Implications for nerve regeneration.
Ali S, Driscoll HE, Newton VL, Gardiner NJ. Ali S, et al. Exp Neurol. 2014 Nov;261:654-65. doi: 10.1016/j.expneurol.2014.08.017. Epub 2014 Aug 23. Exp Neurol. 2014. PMID: 25158309 Free PMC article. - The effect of non-enzymatic glycation of extracellular matrix proteins on axonal regeneration in vitro.
Oztürk G, Sekeroğlu MR, Erdoğan E, Oztürk M. Oztürk G, et al. Acta Neuropathol. 2006 Nov;112(5):627-32. doi: 10.1007/s00401-006-0124-2. Epub 2006 Aug 29. Acta Neuropathol. 2006. PMID: 16941113 - Effects of nonenzymatic glycosylation of extracellular matrix components on cell survival and sensory neurite extension in cell culture.
Luo ZJ, King RH, Lewin J, Thomas PK. Luo ZJ, et al. J Neurol. 2002 Apr;249(4):424-31. doi: 10.1007/s004150200033. J Neurol. 2002. PMID: 11967647 - Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats.
Karachalias N, Babaei-Jadidi R, Ahmed N, Thornalley PJ. Karachalias N, et al. Biochem Soc Trans. 2003 Dec;31(Pt 6):1423-5. doi: 10.1042/bst0311423. Biochem Soc Trans. 2003. PMID: 14641079 Review. - Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options.
Thornalley PJ. Thornalley PJ. Int Rev Neurobiol. 2002;50:37-57. doi: 10.1016/s0074-7742(02)50072-6. Int Rev Neurobiol. 2002. PMID: 12198817 Review.
Cited by
- In-vivo, non-invasive detection of hyperglycemic states in animal models using mm-wave spectroscopy.
Martín-Mateos P, Dornuf F, Duarte B, Hils B, Moreno-Oyervides A, Bonilla-Manrique OE, Larcher F, Krozer V, Acedo P. Martín-Mateos P, et al. Sci Rep. 2016 Sep 27;6:34035. doi: 10.1038/srep34035. Sci Rep. 2016. PMID: 27669659 Free PMC article. - Advanced glycation end products and oxidative stress in type 2 diabetes mellitus.
Nowotny K, Jung T, Höhn A, Weber D, Grune T. Nowotny K, et al. Biomolecules. 2015 Mar 16;5(1):194-222. doi: 10.3390/biom5010194. Biomolecules. 2015. PMID: 25786107 Free PMC article. Review. - Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation.
Li XH, Xie JZ, Jiang X, Lv BL, Cheng XS, Du LL, Zhang JY, Wang JZ, Zhou XW. Li XH, et al. Neuromolecular Med. 2012 Dec;14(4):338-48. doi: 10.1007/s12017-012-8191-0. Epub 2012 Jul 14. Neuromolecular Med. 2012. PMID: 22798221 - Advanced glycation end-products are associated with diabetic neuropathy in young adults with type 1 diabetes.
Al-Saoudi E, Christensen MMB, Nawroth P, Fleming T, Hommel EE, Jørgensen ME, Fleischer J, Hansen CS. Al-Saoudi E, et al. Front Endocrinol (Lausanne). 2022 Oct 11;13:891442. doi: 10.3389/fendo.2022.891442. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36303871 Free PMC article. - Peripheral Neuropathy as a Component of Skeletal Disease in Diabetes.
Beeve AT, Brazill JM, Scheller EL. Beeve AT, et al. Curr Osteoporos Rep. 2019 Oct;17(5):256-269. doi: 10.1007/s11914-019-00528-8. Curr Osteoporos Rep. 2019. PMID: 31392667 Free PMC article. Review.
References
- Letourneau PC, Shattuck TA: Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development 1989; 105: 505– 519 - PubMed
- Condic ML, Letourneau PC: Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature 1997; 389: 852– 856 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources