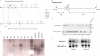Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion - PubMed (original) (raw)
. 2009 Oct 23;284(43):29470-9.
doi: 10.1074/jbc.M109.044396. Epub 2009 Sep 1.
Penghong Song, Suguru Nakamura, Marian Miller, Sharon Barone, Seth L Alper, Brigitte Riederer, Janina Bonhagen, Lois J Arend, Hassane Amlal, Ursula Seidler, Manoocher Soleimani
Affiliations
- PMID: 19723628
- PMCID: PMC2785580
- DOI: 10.1074/jbc.M109.044396
Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion
Jie Xu et al. J Biol Chem. 2009.
Abstract
SLC26A7 (human)/Slc26a7 (mouse) is a recently identified chloride-base exchanger and/or chloride transporter that is expressed on the basolateral membrane of acid-secreting cells in the renal outer medullary collecting duct (OMCD) and in gastric parietal cells. Here, we show that mice with genetic deletion of Slc26a7 expression develop distal renal tubular acidosis, as manifested by metabolic acidosis and alkaline urine pH. In the kidney, basolateral Cl(-)/HCO3(-) exchange activity in acid-secreting intercalated cells in the OMCD was significantly decreased in hypertonic medium (a normal milieu for the medulla) but was reduced only mildly in isotonic medium. Changing from a hypertonic to isotonic medium (relative hypotonicity) decreased the membrane abundance of Slc26a7 in kidney cells in vivo and in vitro. In the stomach, stimulated acid secretion was significantly impaired in isolated gastric mucosa and in the intact organ. We propose that SLC26A7 dysfunction should be investigated as a potential cause of unexplained distal renal tubular acidosis or decreased gastric acid secretion in humans.
Figures
FIGURE 1.
Generation of Slc26a7−/− mutant mice. A, schematic diagram of the Slc26a7 targeting construct. The Neo cassette replaces 4.0 kb of the gene including exons 3 and 4. B, Slc26a7 targeting allele and delineation of the locations of primers. Using primers designated as A1, A2, and A3, which are downstream (3′) to the SA, PCR reactions were performed in conjunction with a primer at the 5′-end of the Neo cassette (referred to as N1). These reactions were expected to amplify 2.1-, 2.2-, and 2.3-kb fragments, respectively. The control PCR reaction was done using AT1 and AT2, which is at the 5′-end of the SA inside the region used to create the targeting construct. This amplifies a band of 2.0 kb. C, identification of homologous recombinant clones. PCR analysis of DNA isolated from 200 surviving colonies identified two individual clones, which showed homologous recombination. Southern blotting confirmed the results. The positive control was performed using primers AT1/N1, which gave the expected fragment size of 1.8 kb. D, generation of Slc26a7+/+ and Slc26a7−/− mice. Tail DNA genotyping identified Slc26a7+/+, +/−, and −/− mice. E, expression of Slc26a7 in kidney and stomach. Crossing of male and female heterozygote mice (+/−) resulted in the generation of Slc26a7 ko (−/−) mice. Northern hybridization on RNA isolated from kidneys and stomachs of Slc26a7 +/+, +/−, and −/− mice indicated that the expression of Slc26a7 is completely absent in Slc26a7-null mouse. Both male and female Slc26a7−/− mice were fertile.
FIGURE 2.
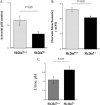
Slc26a7−/− mice have distal renal tubular acidosis. A and B, arterial blood gas and serum bicarbonate. Arterial blood gas and serum chemical analysis demonstrated a significant reduction in arterial pH, and serum bicarbonate, consistent with metabolic acidosis in Slc26a7−/− mice. C, urine pH. Slc26a7 ko mice have elevated urine pH despite presence of metabolic acidosis.
FIGURE 3.
Basolateral Cl−/HCO3− exchanger in acid (A)-intercalated cells in OMCD. A, microperfused mouse kidney OMCD. The acid-secreting (A)-intercalated cells are delineated by high fluorescence intensity reflecting cell type-specific BCECF uptake. B, representative intracellular pH (pH_i_) tracing of an individual A-intercalated cell of OMCD in isotonic solution. Tubules from Slc26a7+/+ (left panel); Slc26a7−/− mice (right panel) were subjected to sequential chloride removal and restoration, first in the absence and then in the presence of DIDS (10 μ
m
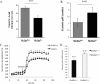
). C, representative intracellular pH (pH_i_) tracing of an individual A-intercalated cell of OMCD in hypertonic solution subjected to the same protocol. Slc26a7+/+ (left panel); Slc26a7−/− (right panel). D, rate of intracellular alkalinization in response to basolateral Cl− removal in A-intercalated cells in isotonic and hypertonic solutions. As shown, basolateral Cl−/HCO3− exchanger activity is significantly decreased in Slc26a7−/− in a hypertonic solution.
FIGURE 4.
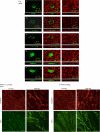
Effect of osmolarity on Slc26a7 expression in vivo and in vitro. Top panel, transient transfection of epitope-tagged Slc26a7 in cultured kidney (MDCK) cells: effect of decreasing tonicity. Cells were transiently transfected with the GFP-SLC26A7 construct in isotonic medium and 24 h later were either exposed to a hypertonic (440 m
m
) medium or remained in isotonic (290 m
m
) medium. Top panel, A, cells incubated in isotonic medium for the duration of transient transfection (48 h) show intracellular localization of Slc26a7. B and C, cells were transfected with GFP-Slc26a7 in isotonic medium and switched to a hypertonic medium 24 h later. 24 h after switching to the hypertonic medium, cells were either switched back to an isotonic (290 m
m
) medium (column C) or remained in hypertonic medium (column B) for an additional 60 min. Cells were fixed and analyzed under confocal microscopy. D and E, cells transfected with the GFP alone (no SLC26A7) and grown in isotonic or hypertonic medium are detected in the cytoplasm (columns D and E). Z-line (side view) images of confocal pictures (lower frame for each column) demonstrate that Slc26a7 is detected predominantly in the basolateral membrane in hypertonic medium (top panel, column B) and intracellularly in isotonic medium either for 48 h (top, column A) or 60 min after being switched back from hypertonic medium (top panel, column C). Frames under each column show side view images. Bottom panel, effect of reduced medullary osmolarity on membrane abundance of Slc26a7 and AE1 in OMCD. Animals were subjected to water loading for 5 days by addition of glucose to their drinking water. Kidney sections from animals with hypertonic medulla (control) or reduced osmolarity (water-loaded) were immunostained with Slc26a7 or AE1 antibodies. Bottom panel, section A, Slc26a7 and AE1 staining in control state. Bottom panel, section B, Slc26a7 and AE1 in water-loaded animals. Slc26a7 shows significant reduction in membrane abundance in water-loaded animals.
FIGURE 5.
Gastric acid secretion in Slc26a7+/+ and Slc26a7−/− mice. Animals 5–6 weeks old were fasted overnight and injected subcutaneously with histamine at 2 μg/g body weight. After 15 min, the intact stomach was removed. The gastric contents, which included both basal and histamine-stimulated acid-base equivalents, were rinsed in 5 ml of normal saline solution and centrifuged. Total acid-base equivalents in the supernatant were determined by titration with NaOH. A, gastric acid secretion in Slc26a7+/+ and Slc26a7−/− mice. Total secreted acid was decreased by ∼36% in Slc26a7−/− versus Slc26a7+/+ mice. B, gastric pH in Slc26a7+/+ and Slc26a7−/− mice. The pH of the gastric secretions was more alkaline in Slc26a7−/− mice. C and D, acid secretory rates in isolated gastric mucosa of adult Slc26a7−/− and Slc26a7+/+ mice. C, acid secretion was measured at basal state and following the stimulation with forskolin (10−5
m
) in gastric mucosa of 40–45 days old (left and right panels). D, peak secretory acid secretion was decreased in Slc26a7−/− mice relative to Slc26a7+/+ mucosa (n = 6 for +/+, 7 for −/− mice).
FIGURE 6.
Schematic diagrams depicting Slc26a7 as a major regulator of acid secretion in the kidney outer medullary collecting duct (A) and stomach parietal cells (B). A, Slc26a7 and AE1 co-localize on the basolateral membrane of acid-secreting intercalated cells in the OMCD. Slc26a7 is predominantly active in hypertonic environment whereas AE1 can function better at isotonic or hypotonic environment. B, Slc26a7 can regulate acid secretion in the stomach by either functioning as a Cl−/HCO3− exchanger (right panel) or an chloride channel (left panel) on the basolateral membrane of gastric parietal cells.
Similar articles
- Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion.
Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, Soleimani M. Xu J, et al. J Am Soc Nephrol. 2006 Apr;17(4):956-67. doi: 10.1681/ASN.2005111174. Epub 2006 Mar 8. J Am Soc Nephrol. 2006. PMID: 16524946 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalised and unclassified epilepsy: two SANAD II non-inferiority RCTs.
Marson AG, Burnside G, Appleton R, Smith D, Leach JP, Sills G, Tudur-Smith C, Plumpton CO, Hughes DA, Williamson PR, Baker G, Balabanova S, Taylor C, Brown R, Hindley D, Howell S, Maguire M, Mohanraj R, Smith PE. Marson AG, et al. Health Technol Assess. 2021 Dec;25(75):1-134. doi: 10.3310/hta25750. Health Technol Assess. 2021. PMID: 34931602 Clinical Trial. - Pharmacological treatments in panic disorder in adults: a network meta-analysis.
Guaiana G, Meader N, Barbui C, Davies SJ, Furukawa TA, Imai H, Dias S, Caldwell DM, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A, Dawson S, Robertson L. Guaiana G, et al. Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
- SLC26 Cl-/HCO3- exchangers in the kidney: roles in health and disease.
Soleimani M. Soleimani M. Kidney Int. 2013 Oct;84(4):657-66. doi: 10.1038/ki.2013.138. Epub 2013 May 1. Kidney Int. 2013. PMID: 23636174 Free PMC article. Review. - The iodide transporter Slc26a7 impacts thyroid function more strongly than Slc26a4 in mice.
Yamaguchi N, Suzuki A, Yoshida A, Tanaka T, Aoyama K, Oishi H, Hara Y, Ogi T, Amano I, Kameo S, Koibuchi N, Shibata Y, Ugawa S, Mizuno H, Saitoh S. Yamaguchi N, et al. Sci Rep. 2022 Jul 4;12(1):11259. doi: 10.1038/s41598-022-15151-4. Sci Rep. 2022. PMID: 35788623 Free PMC article. - Screening and function discussion of a hereditary renal tubular acidosis family pathogenic gene.
Chen L, Wang HL, Zhu YB, Jin Z, Huang JB, Lin XF, Luo JW, Fang ZT. Chen L, et al. Cell Death Dis. 2020 Mar 2;11(3):159. doi: 10.1038/s41419-020-2354-y. Cell Death Dis. 2020. PMID: 32123165 Free PMC article. - High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes.
Brommage R, Liu J, Hansen GM, Kirkpatrick LL, Potter DG, Sands AT, Zambrowicz B, Powell DR, Vogel P. Brommage R, et al. Bone Res. 2014 Oct 28;2:14034. doi: 10.1038/boneres.2014.34. eCollection 2014. Bone Res. 2014. PMID: 26273529 Free PMC article. - Deletion of the Cl-/HCO3- exchanger pendrin downregulates calcium-absorbing proteins in the kidney and causes calcium wasting.
Barone S, Amlal H, Xu J, Soleimani M. Barone S, et al. Nephrol Dial Transplant. 2012 Apr;27(4):1368-79. doi: 10.1093/ndt/gfr505. Epub 2011 Aug 26. Nephrol Dial Transplant. 2012. PMID: 21873623 Free PMC article.
References
- Schuster V. L. (1993) Annu. Rev. Physiol. 55, 267–288 - PubMed
- Weiner I. D., Wingo C. S., Hamm L. L. (1993) Am. J. Physiol. 265, F406–F415 - PubMed
- Karet F. E. (2002) J. Am. Soc. Nephrol. 13, 2178–2184 - PubMed
- Rabon E., Cuppoletti J., Malinowska D., Smolka A., Helander H. F., Mendlein J., Sachs G. (1983) J Exp. Biol. 106, 119–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 43495/DK/NIDDK NIH HHS/United States
- DK62809/DK/NIDDK NIH HHS/United States
- R01 DK062809/DK/NIDDK NIH HHS/United States
- R37 DK043495/DK/NIDDK NIH HHS/United States
- R56 DK062809/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases