The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research - PubMed (original) (raw)
The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research
Martin A Cheever et al. Clin Cancer Res. 2009.
Abstract
The purpose of the National Cancer Institute pilot project to prioritize cancer antigens was to develop a well-vetted, priority-ranked list of cancer vaccine target antigens based on predefined and preweighted objective criteria. An additional aim was for the National Cancer Institute to test a new approach for prioritizing translational research opportunities based on an analytic hierarchy process for dealing with complex decisions. Antigen prioritization involved developing a list of "ideal" cancer antigen criteria/characteristics, assigning relative weights to those criteria using pairwise comparisons, selecting 75 representative antigens for comparison and ranking, assembling information on the predefined criteria for the selected antigens, and ranking the antigens based on the predefined, preweighted criteria. Using the pairwise approach, the result of criteria weighting, in descending order, was as follows: (a) therapeutic function, (b) immunogenicity, (c) role of the antigen in oncogenicity, (d) specificity, (e) expression level and percent of antigen-positive cells, (f) stem cell expression, (g) number of patients with antigen-positive cancers, (h) number of antigenic epitopes, and (i) cellular location of antigen expression. None of the 75 antigens had all of the characteristics of the ideal cancer antigen. However, 46 were immunogenic in clinical trials and 20 of them had suggestive clinical efficacy in the "therapeutic function" category. These findings reflect the current status of the cancer vaccine field, highlight the possibility that additional organized efforts and funding would accelerate the development of therapeutically effective cancer vaccines, and accentuate the need for prioritization.
Figures
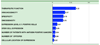
Figure 1
Criteria for an idea cancer antigen were weighted by pair-wise comparison and the resulting relative weights are indicated. Therapeutic function was considered the most important criteria, and was more than twice (0.32/0.15) as important as specificity or oncogenicity.
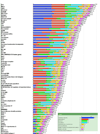
Figure 2
Cancer antigen pilot prioritization: representation of ranking based on predefined and preweighted criteria and sub-criteria. Inset indicates the color used to designate each criterion and its relative weight. The number at the end of each bar indicates the relative rank of that antigen.
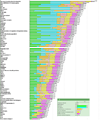
Figure 3
Representation of ranking following exclusion of “therapeutic efficacy” and “immunogenicity”. Inset indicates the color used to designate each criterion and its relative weight. The number at the end of each bar indicates the relative rank of that antigen.
Comment in
- Prioritization of cancer antigens: keeping the target in sight.
Lang JM, Andrei AC, McNeel DG. Lang JM, et al. Expert Rev Vaccines. 2009 Dec;8(12):1657-61. doi: 10.1586/erv.09.134. Expert Rev Vaccines. 2009. PMID: 19943761 No abstract available.
Similar articles
- MAGE-A3: an immunogenic target used in clinical practice.
Esfandiary A, Ghafouri-Fard S. Esfandiary A, et al. Immunotherapy. 2015;7(6):683-704. doi: 10.2217/imt.15.29. Epub 2015 Jun 23. Immunotherapy. 2015. PMID: 26100270 Review. - Leveraging mRNA technology for antigen based immuno-oncology therapies.
Floudas CS, Sarkizova S, Ceccarelli M, Zheng W. Floudas CS, et al. J Immunother Cancer. 2025 Jan 22;13(1):e010569. doi: 10.1136/jitc-2024-010569. J Immunother Cancer. 2025. PMID: 39848687 Free PMC article. Review. - The Landmark Series: Cancer Vaccines for Solid Tumors.
Ninmer EK, Xu F, Slingluff CL Jr. Ninmer EK, et al. Ann Surg Oncol. 2025 Mar;32(3):1443-1452. doi: 10.1245/s10434-024-16712-9. Epub 2024 Dec 20. Ann Surg Oncol. 2025. PMID: 39704984 Free PMC article. Review. - Advances in the development of a therapeutic cancer vaccine.
Avigan D. Avigan D. J Natl Compr Canc Netw. 2005 Nov;3 Suppl 1:S2-6. J Natl Compr Canc Netw. 2005. PMID: 16280106 No abstract available. - Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers.
Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N. Salmaninejad A, et al. Immunol Invest. 2016 Oct;45(7):619-40. doi: 10.1080/08820139.2016.1197241. Epub 2016 Sep 7. Immunol Invest. 2016. PMID: 27603913 Review.
Cited by
- The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer.
Olson BM, Johnson LE, McNeel DG. Olson BM, et al. Cancer Immunol Immunother. 2013 Mar;62(3):585-96. doi: 10.1007/s00262-012-1363-9. Epub 2012 Oct 30. Cancer Immunol Immunother. 2013. PMID: 23108626 Free PMC article. - Dendritic cell vaccination in combination with erlotinib in a patient with inoperable lung adenocarcinoma: a case report.
Kosumi T, Kobayashi M, Shimodaira S, Sugiyama H, Koido S. Kosumi T, et al. J Med Case Rep. 2024 Feb 10;18(1):88. doi: 10.1186/s13256-024-04363-z. J Med Case Rep. 2024. PMID: 38336778 Free PMC article. - Tumor-associated antigens in breast cancer.
Criscitiello C. Criscitiello C. Breast Care (Basel). 2012 Aug;7(4):262-6. doi: 10.1159/000342164. Breast Care (Basel). 2012. PMID: 23904827 Free PMC article. - Design of universal cancer vaccines using natural tumor vessel-specific antigens (SANTAVAC).
Lokhov PG, Balashova EE. Lokhov PG, et al. Hum Vaccin Immunother. 2015;11(3):689-98. doi: 10.1080/21645515.2015.1011022. Hum Vaccin Immunother. 2015. PMID: 25714389 Free PMC article. Review. - The epigenetic factor BORIS (CTCFL) controls the androgen receptor regulatory network in ovarian cancer.
Salgado-Albarrán M, González-Barrios R, Guerra-Calderas L, Alcaraz N, Estefanía Sánchez-Correa T, Castro-Hernández C, Sánchez-Pérez Y, Aréchaga-Ocampo E, García-Carrancá A, Cantú de León D, Herrera LA, Baumbach J, Soto-Reyes E. Salgado-Albarrán M, et al. Oncogenesis. 2019 Aug 12;8(8):41. doi: 10.1038/s41389-019-0150-2. Oncogenesis. 2019. PMID: 31406110 Free PMC article.
References
- Oka Y, Tsuboi A, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Curr Opin Immunol. 2008;20:211–220. - PubMed
- Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat Clin Pract Oncol. 2005;2:138–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- UL1 RR 025014-01/RR/NCRR NIH HHS/United States
- P30 CA 15704-35/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources