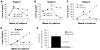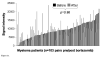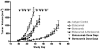Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma - PubMed (original) (raw)
Clinical Trial
doi: 10.1158/1535-7163.MCT-09-0483. Epub 2009 Sep 1.
Susann M Szmania, Myles Dillon, Anne M van Abbema, Xin Li, Mary K Stone, Tarun K Garg, JuMei Shi, Amberly M Moreno-Bost, Rui Yun, Balaji Balasa, Bishwa Ganguly, Debra Chao, Audie G Rice, Fenghuang Zhan, John D Shaughnessy Jr, Bart Barlogie, Shmuel Yaccoby, Daniel E H Afar
Affiliations
- PMID: 19723891
- PMCID: PMC2748787
- DOI: 10.1158/1535-7163.MCT-09-0483
Clinical Trial
Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma
Frits van Rhee et al. Mol Cancer Ther. 2009 Sep.
Abstract
Monoclonal antibody (mAb) therapy for multiple myeloma, a malignancy of plasma cells, has not been clinically efficacious in part due to a lack of appropriate targets. We recently reported that the cell surface glycoprotein CS1 (CD2 subset 1, CRACC, SLAMF7, CD319) was highly and universally expressed on myeloma cells while having restricted expression in normal tissues. Elotuzumab (formerly known as HuLuc63), a humanized mAb targeting CS1, is currently in a phase I clinical trial in relapsed/refractory myeloma. In this report we investigated whether the activity of elotuzumab could be enhanced by bortezomib, a reversible proteasome inhibitor with significant activity in myeloma. We first showed that elotuzumab could induce patient-derived myeloma cell killing within the bone marrow microenvironment using a SCID-hu mouse model. We next showed that CS1 gene and cell surface protein expression persisted on myeloma patient-derived plasma cells collected after bortezomib administration. In vitro bortezomib pretreatment of myeloma targets significantly enhanced elotuzumab-mediated antibody-dependent cell-mediated cytotoxicity, both for OPM2 myeloma cells using natural killer or peripheral blood mononuclear cells from healthy donors and for primary myeloma cells using autologous natural killer effector cells. In an OPM2 myeloma xenograft model, elotuzumab in combination with bortezomib exhibited significantly enhanced in vivo antitumor activity. These findings provide the rationale for a clinical trial combining elotuzumab and bortezomib, which will test the hypothesis that combining both drugs would result in enhanced immune lysis of myeloma by elotuzumab and direct targeting of myeloma by bortezomib.
Conflict of interest statement
Conflict-of-interest disclosure DEHA, MD, AvA, YR, B. Balasa, BG, AGR, and DC are current or former employees at Facet Biotech Corporation (formerly PDL BioPharma), FvR has received research funding from PDL Biopharma, B. Barlogie has received research funding from Millenium Pharmaceuticals.
Figures
Figure 1. Anti-CS1 mAb MuLuc63 induces marked reduction of primary human myeloma tumors in the SCID hu host
A-D show each individual experiment, each using patient derived myeloma injected into an implanted human bone allowed to grow, then treated with anti-CS1 mAb MuLuc63 or murine isotype control mIgG2a ab. A dramatic reduction in tumor load was observed in each experiment. E. The difference of hu Ig detected (calculated as the % of pre treatment) for MuLuc63 treated versus control mIgG2a mAb is significantly different (student's t test).
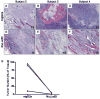
Figure 2. Histological analysis of tumor burden with anti-CS1 therapy
The tumor burden in isotype control (mIgG2a) treated mice versus MuLuc63 treated mice was calculated as the percent of tissue comprised of myeloma cells and is shown for the three evaluable subjects. Histological analysis showed a dramatic reduction in tumor burden in MuLuc63 treated (D-F) versus isotype control treated mice (A-C). G. The tumor burden was scored by a certified pathologist.
Figure 3. CS1 gene expression persists in patients with MM after bortezomib treatment
A. CS1 gene expression profiling of CD138 positive myeloma cells purified from bone marrow before and 48 hours after administration of 1mg/m2 bortezomib. Paired signal intensities are shown for 103 newly diagnosed myeloma patients. The student's t test was performed to determine whether differences were significantly different. B. Flow cytometry analysis of purified CD138 positive cells demonstrated that cell surface CS1 expression remains present 48 hrs post bortezomib. A gate was applied which only captured Annexin V and PI negative CD138 purified myeloma cells.
Figure 3. CS1 gene expression persists in patients with MM after bortezomib treatment
A. CS1 gene expression profiling of CD138 positive myeloma cells purified from bone marrow before and 48 hours after administration of 1mg/m2 bortezomib. Paired signal intensities are shown for 103 newly diagnosed myeloma patients. The student's t test was performed to determine whether differences were significantly different. B. Flow cytometry analysis of purified CD138 positive cells demonstrated that cell surface CS1 expression remains present 48 hrs post bortezomib. A gate was applied which only captured Annexin V and PI negative CD138 purified myeloma cells.
Figure 4. Bortezomib pretreatment enhances elotuzumab-mediated ADCC
A) Results are shown for ADCC assays conducted with NK cells from 4 separate healthy donors against the OPM2 myeloma cell line. Results are expressed as mean ± SD. B) Results are shown for ADCC assays using PBMC effectors from a healthy donor versus the myeloma cell lines XG-1 and RPMI8226/R5 at a ratio of 40 effectors:1 target cell. Results are expressed as the mean with SD. *Student's Ttest determined that lysis of XG1 targets treated with both elotuzumab and bortezomib was significantly higher (p<0.006) than elotuzumab or bortezomib alone. C) Results are shown for reactions conducted with PBMCs from six healthy individuals against OPM2 cells which were incubated for 18 hr with vehicle, 5 nM (upper panel) or 1 nM (lower panel) bortezomib as indicated. Results (% Cytotoxicity) are expressed as mean ± SD. A dose of 5 nM bortezomib consistently enhanced elotuzumab-mediated ADCC compared to vehicle treatment (p = 0.0057). A dose of 1 nM bortezomib was not significantly different from vehicle (p = 0.731). D) Results are shown for reactions conducted with patient derived NK cells against autologous myeloma cells which were incubated for 18h with vehicle (dotted lines), 5nM bortezomib (dashed/dotted lines) or 20nM bortezomib (solid lines). Targets were also incubated with either isotype control mAb (□) or Elotuzumab (■) during the assay. K562 cells (△) were included as a positive control. Results are expressed as mean ± SEM.
Figure 4. Bortezomib pretreatment enhances elotuzumab-mediated ADCC
A) Results are shown for ADCC assays conducted with NK cells from 4 separate healthy donors against the OPM2 myeloma cell line. Results are expressed as mean ± SD. B) Results are shown for ADCC assays using PBMC effectors from a healthy donor versus the myeloma cell lines XG-1 and RPMI8226/R5 at a ratio of 40 effectors:1 target cell. Results are expressed as the mean with SD. *Student's Ttest determined that lysis of XG1 targets treated with both elotuzumab and bortezomib was significantly higher (p<0.006) than elotuzumab or bortezomib alone. C) Results are shown for reactions conducted with PBMCs from six healthy individuals against OPM2 cells which were incubated for 18 hr with vehicle, 5 nM (upper panel) or 1 nM (lower panel) bortezomib as indicated. Results (% Cytotoxicity) are expressed as mean ± SD. A dose of 5 nM bortezomib consistently enhanced elotuzumab-mediated ADCC compared to vehicle treatment (p = 0.0057). A dose of 1 nM bortezomib was not significantly different from vehicle (p = 0.731). D) Results are shown for reactions conducted with patient derived NK cells against autologous myeloma cells which were incubated for 18h with vehicle (dotted lines), 5nM bortezomib (dashed/dotted lines) or 20nM bortezomib (solid lines). Targets were also incubated with either isotype control mAb (□) or Elotuzumab (■) during the assay. K562 cells (△) were included as a positive control. Results are expressed as mean ± SEM.
Figure 4. Bortezomib pretreatment enhances elotuzumab-mediated ADCC
A) Results are shown for ADCC assays conducted with NK cells from 4 separate healthy donors against the OPM2 myeloma cell line. Results are expressed as mean ± SD. B) Results are shown for ADCC assays using PBMC effectors from a healthy donor versus the myeloma cell lines XG-1 and RPMI8226/R5 at a ratio of 40 effectors:1 target cell. Results are expressed as the mean with SD. *Student's Ttest determined that lysis of XG1 targets treated with both elotuzumab and bortezomib was significantly higher (p<0.006) than elotuzumab or bortezomib alone. C) Results are shown for reactions conducted with PBMCs from six healthy individuals against OPM2 cells which were incubated for 18 hr with vehicle, 5 nM (upper panel) or 1 nM (lower panel) bortezomib as indicated. Results (% Cytotoxicity) are expressed as mean ± SD. A dose of 5 nM bortezomib consistently enhanced elotuzumab-mediated ADCC compared to vehicle treatment (p = 0.0057). A dose of 1 nM bortezomib was not significantly different from vehicle (p = 0.731). D) Results are shown for reactions conducted with patient derived NK cells against autologous myeloma cells which were incubated for 18h with vehicle (dotted lines), 5nM bortezomib (dashed/dotted lines) or 20nM bortezomib (solid lines). Targets were also incubated with either isotype control mAb (□) or Elotuzumab (■) during the assay. K562 cells (△) were included as a positive control. Results are expressed as mean ± SEM.
Figure 4. Bortezomib pretreatment enhances elotuzumab-mediated ADCC
A) Results are shown for ADCC assays conducted with NK cells from 4 separate healthy donors against the OPM2 myeloma cell line. Results are expressed as mean ± SD. B) Results are shown for ADCC assays using PBMC effectors from a healthy donor versus the myeloma cell lines XG-1 and RPMI8226/R5 at a ratio of 40 effectors:1 target cell. Results are expressed as the mean with SD. *Student's Ttest determined that lysis of XG1 targets treated with both elotuzumab and bortezomib was significantly higher (p<0.006) than elotuzumab or bortezomib alone. C) Results are shown for reactions conducted with PBMCs from six healthy individuals against OPM2 cells which were incubated for 18 hr with vehicle, 5 nM (upper panel) or 1 nM (lower panel) bortezomib as indicated. Results (% Cytotoxicity) are expressed as mean ± SD. A dose of 5 nM bortezomib consistently enhanced elotuzumab-mediated ADCC compared to vehicle treatment (p = 0.0057). A dose of 1 nM bortezomib was not significantly different from vehicle (p = 0.731). D) Results are shown for reactions conducted with patient derived NK cells against autologous myeloma cells which were incubated for 18h with vehicle (dotted lines), 5nM bortezomib (dashed/dotted lines) or 20nM bortezomib (solid lines). Targets were also incubated with either isotype control mAb (□) or Elotuzumab (■) during the assay. K562 cells (△) were included as a positive control. Results are expressed as mean ± SEM.
Figure 5. Bortezomib enhances elotuzumab-mediated antitumor activity in the OPM2 mouse xenograft model
OPM2 tumor-bearing mice (4 groups of n = 15 each) were treated with elotuzumab + bortezomib, bortezomib + isotype control mAb, PBS + elotuzumab, and PBS + isotype control mAb. Sub-optimal doses of elotuzumab (1 mg/kg) or isotype control were administered intraperitoneally twice weekly and bortezomib 1 mg/kg was administered intraperitoneally twice weekly. Tumor volumes are expressed as mean ± SD.
Similar articles
- Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.
Campbell KS, Cohen AD, Pazina T. Campbell KS, et al. Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review. - CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma.
Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G, Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S, Vexler V, Shaughnessy JD Jr, Barlogie B, van Rhee F, Hussein M, Afar DE, Williams MB. Hsi ED, et al. Clin Cancer Res. 2008 May 1;14(9):2775-84. doi: 10.1158/1078-0432.CCR-07-4246. Clin Cancer Res. 2008. PMID: 18451245 Free PMC article. - Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways.
Balasa B, Yun R, Belmar NA, Fox M, Chao DT, Robbins MD, Starling GC, Rice AG. Balasa B, et al. Cancer Immunol Immunother. 2015 Jan;64(1):61-73. doi: 10.1007/s00262-014-1610-3. Epub 2014 Oct 7. Cancer Immunol Immunother. 2015. PMID: 25287778 Free PMC article. - Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu.
Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. Tai YT, et al. Blood. 2008 Aug 15;112(4):1329-37. doi: 10.1182/blood-2007-08-107292. Epub 2007 Sep 28. Blood. 2008. PMID: 17906076 Free PMC article. - Efficacy and safety of elotuzumab for the treatment of multiple myeloma.
Gavriatopoulou M, Terpos E, Kastritis E, Dimopoulos MA. Gavriatopoulou M, et al. Expert Opin Drug Saf. 2017 Feb;16(2):237-245. doi: 10.1080/14740338.2017.1279603. Epub 2017 Jan 11. Expert Opin Drug Saf. 2017. PMID: 28060563 Review.
Cited by
- Spotlight on elotuzumab in the treatment of multiple myeloma: the evidence to date.
Weisel K. Weisel K. Onco Targets Ther. 2016 Oct 5;9:6037-6048. doi: 10.2147/OTT.S94531. eCollection 2016. Onco Targets Ther. 2016. PMID: 27785050 Free PMC article. Review. - Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM.
Jakubowiak A, Offidani M, Pégourie B, De La Rubia J, Garderet L, Laribi K, Bosi A, Marasca R, Laubach J, Mohrbacher A, Carella AM, Singhal AK, Tsao LC, Lynch M, Bleickardt E, Jou YM, Robbins M, Palumbo A. Jakubowiak A, et al. Blood. 2016 Jun 9;127(23):2833-40. doi: 10.1182/blood-2016-01-694604. Epub 2016 Apr 18. Blood. 2016. PMID: 27091875 Free PMC article. Clinical Trial. - Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.
Campbell KS, Cohen AD, Pazina T. Campbell KS, et al. Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review. - Development of target-specific treatments in multiple myeloma.
Chanan-Khan AA, Borrello I, Lee KP, Reece DE. Chanan-Khan AA, et al. Br J Haematol. 2010 Oct;151(1):3-15. doi: 10.1111/j.1365-2141.2010.08262.x. Epub 2010 Jul 7. Br J Haematol. 2010. PMID: 20618339 Free PMC article. Review. - Immunotherapeutics in Multiple Myeloma: How Can Translational Mouse Models Help?
Cooke RE, Koldej R, Ritchie D. Cooke RE, et al. J Oncol. 2019 Apr 10;2019:2186494. doi: 10.1155/2019/2186494. eCollection 2019. J Oncol. 2019. PMID: 31093282 Free PMC article. Review.
References
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. see comment. - PubMed
- Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–85. - PubMed
- Moreau P, Voillat L, Benboukher L, et al. Rituximab in CD20 positive multiple myeloma. Leukemia. 2007;21:835–6. - PubMed
- Zojer N, Kirchbacher K, Vesely M, Hubl W, Ludwig H. Rituximab treatment provides no clinical benefit in patients with pretreated advanced multiple myeloma. Leuk Lymph. 2006;47:1103–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA134522-02/CA/NCI NIH HHS/United States
- CA134522/CA/NCI NIH HHS/United States
- R01 CA134522/CA/NCI NIH HHS/United States
- R01 CA134522-01/CA/NCI NIH HHS/United States
- CA55819/CA/NCI NIH HHS/United States
- R01 CA093897-06/CA/NCI NIH HHS/United States
- P01 CA055819/CA/NCI NIH HHS/United States
- R01 CA093897/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous