NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses - PubMed (original) (raw)
Comparative Study
NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses
Kaori Akashi et al. J Neurosci. 2009.
Abstract
GluN2B (GluRepsilon2/NR2B) subunit is involved in synapse development, synaptic plasticity, and cognitive function. However, its roles in synaptic expression and function of NMDA receptors (NMDARs) in the brain remain mostly unknown because of the neonatal lethality of global knock-out mice. To address this, we generated conditional knock-out mice, in which GluN2B was ablated exclusively in hippocampal CA3 pyramidal cells. By immunohistochemistry, GluN2B disappeared and GluN1 (GluRzeta1/NR1) was moderately reduced, whereas GluN2A (GluRepsilon1/NR2A) and postsynaptic density-95 (PSD-95) were unaltered in the mutant CA3. This was consistent with protein contents in the CA3 crude fraction: 9.6% of control level for GluN2B, 47.7% for GluN1, 90.6% for GluN2A, and 98.0% for PSD-95. Despite the remaining NMDARs, NMDAR-mediated currents and long-term potentiation were virtually lost at various CA3 synapses. Then, we compared synaptic NMDARs by postembedding immunogold electron microscopy and immunoblot using the PSD fraction. In the mutant CA3, GluN1 was severely reduced in both immunogold (20.6-23.6%) and immunoblot (24.6%), whereas GluN2A and PSD-95 were unchanged in immunogold but markedly reduced in the PSD fraction (51.4 and 36.5%, respectively), indicating increased detergent solubility of PSD molecules. No such increased solubility was observed for GluN2B in the CA3 of GluN2A-knock-out mice. Furthermore, significant decreases were found in the ratio of filamentous to globular actin (49.5%) and in the density of dendritic spines (76.2%). These findings suggest that GluN2B is critically involved in NMDAR channel function, organization of postsynaptic macromolecular complexes, formation or maintenance of dendritic spines, and regulation of the actin cytoskeleton.
Figures
Figure 1.
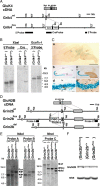
Generation of Grin2b-flox and Grik4-Cre mice. A, Schematic representations of GluK4 cDNA, genomic DNA (Grik4wt), and targeted genome (Grik4Cre). The gray boxes indicate exons. The black boxes indicate the probes for Southern blot analysis. The filled circles in the Grik4Cre allele delineate the 5′ and 3′ termini of the targeting vector. The semicircles indicate FRT sequences. Met, Initial methionine; Cre, Cre recombinase gene; neo, neomycin-resistant gene expression cassette; Xb, _Xba_I; ET, _Eco_T22-I. B, Southern blot analysis of _Xba_I- or _Eco_T22-I-digested genomic DNA prepared from the wild-type (Grik4+/+) and heterozygous Cre (Grik4Cre/+) mice. C, β-Galactosidase activity induced by Grik4-Cre in 4-week-old reporter mice. a, β-Galactosidase staining of a whole-brain slice (50 μm thick). b, c, Hippocampus, counterstained with nuclear fast red (NFR) (b) or reacted with anti-calbindin antibody (c). d, e, CA3 region. Parvalbumin (d) and glutamine synthase (e) were stained with respective antibodies as markers for interneurons and glial cells, respectively. Scale bars: a, 3 mm; b, c, 100 μm; d, e, 10 μm. D, Schematic representations of GluN2B cDNA, genomic DNA (Grin2bwt), targeted genome (Grin2bflox), and Cre-mediated deleted genome (Grin2bdel). The gray boxes indicate exons. The black boxes indicate the probes for Southern blot analysis. The triangles indicate loxP sequences. The filled circles in the Grin2bflox allele delineate the 5′ and 3′ termini of the targeting vector. Nh, _Nhe_I; Nd, _Nde_I. E, Southern blot analysis of _Nde_I- or Nhe_I-digested genomic DNA prepared from the wild-type (Grin2b+/+), heterozygous floxed (Grin2b+/flox), homozygous floxed (Grin2bflox/flox), and heterozygous floxed and deleted (Grin2bflox/del) mice. F, Immunoblot analysis of whole-brain crude fraction prepared from the wild-type, heterozygous (Grin2b+/flox), and homozygous floxed (Grin2bflox/flox) mice at P0.5 with anti-GluN2B_C and anti-NSE antibodies.
Figure 2.
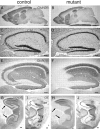
Selective deletion of GluN2B subunit in CA3 pyramidal cells of CA3-GluN2B-KO mice. Immunohistochemistry for GluN2B in mutant and control mice at 2 months of age. Immunoreactivities for GluN2B in the whole brain (A, B), hippocampus (E, F), and the dorsal and ventral hippocampi (G, H). Normal histology of the hippocampus in mutant mice (C, D). Immunoreactivities for calbindin used as a marker for subfields of the hippocampal formation (I, J): dense somal and dendritic labeling in the dentate gyrus (DG), dense mossy fiber labeling in the CA3 (CA3), and low diffuse labeling in the CA1. The arrows in G–J indicate the CA3 subfield. CB, Calbindin; dH, dorsal hippocampus; vH, ventral hippocampus. Scale bars: A, B, 1 mm; C–J, 0.1 mm.
Figure 3.
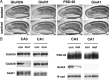
Effects of CA3 region-selective GluN2B deletion on PSD-associated proteins. A, Immunohistochemistry for GluN2A, GluN1-C2, PSD-95, and GluA1 in the mutant and control hippocampus. Scale bar, 0.1 mm. B, Immunoblot analysis of crude fraction from CA1 and CA3 of control and mutant mice with respective antibodies (GluN2B, GluN2A, GluN1, PSD-95, GluK4, and N-cadherin). Each lane was loaded with 20 μg of protein.
Figure 4.
Effects of GluN2B deficiency on NMDAR-mediated EPSCs and LTPs in CA3 area. A, Effects on NMDAR-mediated EPSCs at the synapses in hippocampal CA3 (C/A–CA3, Com–CA3, and MF–CA3) and CA1 (SC–CA1). The upward and downward traces show NMDAR-mediated EPSCs in the presence of CNQX and non-NMDAR-mediated EPSCs in the control solution, respectively. B, Effects on extracellular field potentials after tetanic stimulation (100 Hz, 1 s). The averaged time course of the field EPSP slope. The open and filled circles represent control and mutant slices, respectively.
Figure 5.
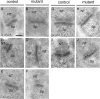
Synaptic localization of NMDA receptor subunits in the stratum radiatum of hippocampal CA3 region. Postembedding immunogold labeling for GluN2A (A, B), GluN2B (C, D), GluN1-C2 (E, F), GluN1-C2′ (G, H), and PSD-95 (I, J) in the stratum radiatum of control and mutant CA3 region. NT, Nerve terminal; Sp, spine. Scale bar, 100 nm.
Figure 6.
NMDAR-mediated currents evoked by glutamate application in CA3-GluN2B-KO mice. A, Currents were evoked by iontophoretic glutamate (Glu) application to the soma or dendrites of CA3 pyramidal cells. The downward and upward traces show non-NMDAR-mediated EPSCs at −80 mV (a) and NMDAR- and/or non-NMDAR-mediated EPSCs at +50 mV (b–d), respectively. B, AMPAR-mediated (b − c) and NMDAR-mediated (c − d) responses.
Figure 7.
Effects of GluN2B deletion on the proteins in PSD fraction from CA3 region. Immunoblots with respective antibodies (GluN2A, GluN2B, GluN1, PSD-95, N-cadherin, GluA1, and SAP97) in PSD fraction from CA3 region of control and mutant. Each lane was loaded with 2 μg of protein.
Figure 8.
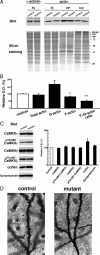
GluN2B ablation decreases F-actin content in CA3 synaptosomes. A, Synaptosomal fraction (Sy) was prepared from hippocampal CA3 region. G-actin and F-actin fractions were obtained from the synaptosomal fraction as NP-40 soluble (sup) and insoluble (ppt) fractions, respectively. In each lane, 10 μg of protein was loaded. Each fraction was separated by SDS-PAGE, and silver-stained (bottom panel) or immunoblotted with an anti-α-actinin or anti-actin antibody. B, Summary graphs indicate relative content of total actin, G-actin, F-actin, and relative ratio of F-actin to G-actin in the mutant to control. **p < 0.03; *p < 0.05. Error bars indicate SEM. C, Immunoblots with antibodies to CaMKIIα, phospho-Thr286-CaMKIIα, CaMKIIβ, phospho-Thr287-CaMKIIβ, cofilin, and synaptophysin (left panel). In each lane, 20 μg of protein of CA3 crude fraction was loaded. Summary graphs (right panel) indicate density of immunoreactivity in mutant normalized to that in control for each molecule. D, Photomicrographs showing Golgi-impregnated apical dendrites of CA3 pyramidal cells from control (left panel) and mutant (right panel) mice. Note apparent reduction of dendritic spines in CA3 pyramidal cells of CA3-GluN2B-KO mice. Scale bar, 5 μm.
Similar articles
- Activity-dependent control of NMDA receptor subunit composition at hippocampal mossy fibre synapses.
Carta M, Srikumar BN, Gorlewicz A, Rebola N, Mulle C. Carta M, et al. J Physiol. 2018 Feb 15;596(4):703-716. doi: 10.1113/JP275226. Epub 2018 Jan 30. J Physiol. 2018. PMID: 29218821 Free PMC article. - Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses.
Rauner C, Köhr G. Rauner C, et al. J Biol Chem. 2011 Mar 4;286(9):7558-66. doi: 10.1074/jbc.M110.182600. Epub 2010 Dec 29. J Biol Chem. 2011. PMID: 21190942 Free PMC article. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review. - Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.
MacDonald JF, Jackson MF, Beazely MA. MacDonald JF, et al. Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
- Characterization of Mice Carrying a Neurodevelopmental Disease-Associated GluN2B(L825V) Variant.
Candelas Serra M, Kuchtiak V, Kubik-Zahorodna A, Kysilov B, Fili K, Hrcka Krausova B, Abramova V, Dobrovolski M, Harant K, Bozikova P, Cerny J, Prochazka J, Kasparek P, Sedlacek R, Balik A, Smejkalova T, Vyklicky L. Candelas Serra M, et al. J Neurosci. 2024 Jul 31;44(31):e2291232024. doi: 10.1523/JNEUROSCI.2291-23.2024. J Neurosci. 2024. PMID: 38926089 - Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning.
Snyder MA, Adelman AE, Gao WJ. Snyder MA, et al. Neuropsychopharmacology. 2013 Jan;38(2):328-40. doi: 10.1038/npp.2012.180. Epub 2012 Sep 12. Neuropsychopharmacology. 2013. PMID: 22968815 Free PMC article. - NMDA receptors in GABAergic synapses during postnatal development.
Cserép C, Szabadits E, Szőnyi A, Watanabe M, Freund TF, Nyiri G. Cserép C, et al. PLoS One. 2012;7(5):e37753. doi: 10.1371/journal.pone.0037753. Epub 2012 May 25. PLoS One. 2012. PMID: 22662211 Free PMC article. - The primate-specific peptide Y-P30 regulates morphological maturation of neocortical dendritic spines.
Neumann JR, Dash-Wagh S, Jack A, Räk A, Jüngling K, Hamad MIK, Pape HC, Kreutz MR, Puskarjov M, Wahle P. Neumann JR, et al. PLoS One. 2019 Feb 13;14(2):e0211151. doi: 10.1371/journal.pone.0211151. eCollection 2019. PLoS One. 2019. PMID: 30759095 Free PMC article. - Proteomic Analysis of Postsynaptic Protein Complexes Underlying Neuronal Plasticity.
Baucum AJ 2nd. Baucum AJ 2nd. ACS Chem Neurosci. 2017 Apr 19;8(4):689-701. doi: 10.1021/acschemneuro.7b00008. Epub 2017 Feb 23. ACS Chem Neurosci. 2017. PMID: 28211672 Free PMC article. Review.
References
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;486:384–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous