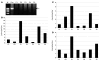Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy - PubMed (original) (raw)
Case Reports
. 2009 Sep;119(9):2623-33.
doi: 10.1172/JCI38660. Epub 2009 Aug 10.
Chie Matsuda, Megumu Ogawa, Kanako Goto, Kayo Tominaga, Satomi Mitsuhashi, Young-Eun Park, Ikuya Nonaka, Naomi Hino-Fukuyo, Kazuhiro Haginoya, Hisashi Sugano, Ichizo Nishino
Affiliations
- PMID: 19726876
- PMCID: PMC2735915
- DOI: 10.1172/JCI38660
Case Reports
Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy
Yukiko K Hayashi et al. J Clin Invest. 2009 Sep.
Abstract
Caveolae are invaginations of the plasma membrane involved in many cellular processes, including clathrin-independent endocytosis, cholesterol transport, and signal transduction. They are characterized by the presence of caveolin proteins. Mutations that cause deficiency in caveolin-3, which is expressed exclusively in skeletal and cardiac muscle, have been linked to muscular dystrophy. Polymerase I and transcript release factor (PTRF; also known as cavin) is a caveolar-associated protein suggested to play an essential role in the formation of caveolae and the stabilization of caveolins. Here, we identified PTRF mutations in 5 nonconsanguineous patients who presented with both generalized lipodystrophy and muscular dystrophy. Muscle hypertrophy, muscle mounding, mild metabolic complications, and elevated serum creatine kinase levels were observed in these patients. Skeletal muscle biopsies revealed chronic dystrophic changes, deficiency and mislocalization of all 3 caveolin family members, and reduction of caveolae structure. We generated expression constructs recapitulating the human mutations; upon overexpression in myoblasts, these mutations resulted in PTRF mislocalization and disrupted physical interaction with caveolins. Our data confirm that PTRF is essential for formation of caveolae and proper localization of caveolins in human cells and suggest that clinical features observed in the patients with PTRF mutations are associated with a secondary deficiency of caveolins.
Figures
Figure 1. Mutations in PTRF.
(A) All 5 patients had a homozygous or compound heterozygous mutation in PTRF (shown by arrows). P1–P4 had the same homozygous insertion mutation of c.696_697insC (InsC) in exon 2, whereas P5 had a compound heterozygous mutation of the same c.696_697insC insertion mutation and a deletion mutation of c.525delG (DelG) in exon 2. (B) Schema of the position of mutations in PTRF, putative proteins produced by mutations, and antibody recognition sites. The c.525delG mutant changes the last 275 amino acids to an unrelated 98–amino acid sequence, while the c.696_697insC mutant substitutes the last 158 amino acids with an unrelated 191–amino acid sequence.
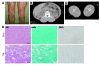
Figure 2. Muscle hypertrophy and dystrophic changes.
(A) Prominent musculature feature of legs in P5. (B and C) CT images from P4 showed hypertrophy of paravertebral and thigh muscles with minimal subcutaneous and intra-abdominal fat tissue. (D) H&E stain of biopsied skeletal muscle from P4 showed dystrophic changes, including marked variation in fiber size, enlarged fibers with internalized nuclei, endomysial fibrosis, and few necrotic and regenerating fibers. Intramuscular lipid droplets were not increased compared with control. mGt, modified Gomori trichrome; ORO, oil red O. Scale bars: 50 μm.
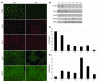
Figure 3. Loss of PTRF is associated with deficiency and mislocalization of caveolins in muscle.
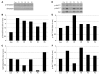
(A) In control muscle, PTRF was clearly seen in sarcolemma as strongly staining blood vessels. Caveolin-3 (Cav3) was clearly visible at sarcolemma, and caveion-1 and -2 stained intramuscular blood vessels. The muscle of P4 was negative for PTRF. Membrane staining of caveolin-3 was reduced with increased cytoplasmic staining, and caveolin-1 and -2 were barely detectable. Immunoreactivity of nNOS varied between muscle fibers, but was not markedly different between control and patient muscle. Scale bar: 50 μm. (B) Immunoblotting analysis of skeletal muscles. 3T3 cells were used as a positive control. PTRF and caveolin-2 were seen only in the muscles of 2 control subjects and in 3T3 cells, and were barely detectable in the muscles of P1–P5. The bands for caveolin-3 and nNOS were variably seen. (C and D) Quantification of immunoreactive bands was performed by densitometric analysis and normalized with MHC. In P1–P5, relative amounts of caveolin-3 decreased compared with control subjects (C), whereas nNOS amounts varied (D).
Figure 4. mRNA expression of PTRF in skeletal muscle, and quantitative RT-PCR of mRNAs for caveolins.
(A) RT-PCR analysis revealed a single band for PTRF mRNA (arrow) in a control subject, but no detectable product was seen in P1–P5. M, marker. (B–D) By quantitative RT-PCR, mRNA for CAV1, CAV2, and CAV3 normalized with GAPDH expression was not decreased in P1–P5.
Figure 5. Reduced caveolae formation in skeletal muscle, as assessed by electron microscopy.
In control muscle, an abundance of caveolae (arrowheads) was observed close to the plasma membrane. Plasma membrane of muscle fibers from P2 and P3 was nearly flat, and caveolae density was greatly reduced compared with that of control muscle. Only a few caveolae were seen in P2. Scale bars: 200 nm.
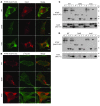
Figure 6. Altered localization of mutant PTRF in C2C12 cells and reduced binding ability to caveolins.
C2C12 myoblasts were cotransfected with FLAG-tagged WT or mutant (c.525delG or c.696_697insC) PTRF cDNA and T7-tagged human caveolin-3. (A and B) WT PTRF stained by anti-FLAG antibody colocalized with caveolin-3 at the cell membrane. The deletion mutant accumulated in the nucleus, and the insertion mutant was seen in cytoplasm. (A) Membrane staining of caveolin-3 was decreased and was not colocalized with mutant PTRF. (B) The PTRF insertion mutant clearly colocalized with β-tubulin. Scale bars: 10 μm. (C and D) COS-7 cells were cotransfected with FLAG-tagged WT or mutant PTRF cDNA and T7-tagged human caveolin-3 (C) or caveolin-1 (D). The PTRF deletion mutant showed smaller molecular weight (estimated 30 kDa), and no immunoprecipitated protein was detected for FLAG or T7 antibodies. The PTRF insertion mutant showed slightly larger molecular weight, and amounts of coimmunoprecipitated proteins were greatly reduced. W, whole homogenate; L, cell lysate, G, control IgG; F, anti-FLAG; T, anti-T7.
Figure 7. Increased p-Smad2 and p-Akt in P1–P5 skeletal muscle.
(A–C) Immunoblotting analysis of Smad2/3 and p-Smad2/3S423/425 (A) and densitometric analysis (B) showed increased p-Smad2/3 in P1–P5 compared with control muscle, with variable mRNA expression levels of myostatin (MSTN; C). (D–F) Immunoblotting analysis of p-AktT308 and p-AktS473. Total Akt (D) and densitometric analysis (E and F) showed increased amounts of p-Akt in all patients except for p-AktS473 in P2.
Figure 8. NDP activity assay.
NDP activity was variable between muscle fibers, and was slightly increased in the muscle of P4, P5, and a LGMD1C patient with CAV3 mutation compared with an age-matched control subject. Scale bars: 100 μm.
Comment in
- Lipodystrophy and muscular dystrophy caused by PTRF mutations.
de Haan W. de Haan W. Clin Genet. 2010 May;77(5):436-7. doi: 10.1111/j.1399-0004.2009.01365_3.x. Clin Genet. 2010. PMID: 20447152
Similar articles
- Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations.
Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, Schulze A, Lucke B, Lützkendorf S, Karbasiyan M, Bachmann S, Spuler S, Schuelke M. Rajab A, et al. PLoS Genet. 2010 Mar 12;6(3):e1000874. doi: 10.1371/journal.pgen.1000874. PLoS Genet. 2010. PMID: 20300641 Free PMC article. - Caveolae and caveolin-3 in muscular dystrophy.
Galbiati F, Razani B, Lisanti MP. Galbiati F, et al. Trends Mol Med. 2001 Oct;7(10):435-41. doi: 10.1016/s1471-4914(01)02105-0. Trends Mol Med. 2001. PMID: 11597517 Review. - A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes.
Smythe GM, Eby JC, Disatnik MH, Rando TA. Smythe GM, et al. J Cell Sci. 2003 Dec 1;116(Pt 23):4739-49. doi: 10.1242/jcs.00806. J Cell Sci. 2003. PMID: 14600260 - Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair.
Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Zhu H, et al. J Biol Chem. 2011 Apr 15;286(15):12820-4. doi: 10.1074/jbc.C111.221440. Epub 2011 Feb 22. J Biol Chem. 2011. PMID: 21343302 Free PMC article. - A new mutation in the CAVIN1/PTRF gene in two siblings with congenital generalized lipodystrophy type 4: case reports and review of the literature.
Mancioppi V, Daffara T, Romanisio M, Ceccarini G, Pelosini C, Santini F, Bellone S, Mellone S, Baricich A, Rabbone I, Aimaretti G, Akinci B, Giordano M, Prodam F. Mancioppi V, et al. Front Endocrinol (Lausanne). 2023 Jul 12;14:1212729. doi: 10.3389/fendo.2023.1212729. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37501786 Free PMC article. Review.
Cited by
- A Patient with Congenital Generalized Lipodystrophy Due To a Novel Mutation in BSCL2: Indications for Secondary Mitochondrial Dysfunction.
Jeninga EH, de Vroede M, Hamers N, Breur JM, Verhoeven-Duif NM, Berger R, Kalkhoven E. Jeninga EH, et al. JIMD Rep. 2012;4:47-54. doi: 10.1007/8904_2011_86. Epub 2011 Nov 4. JIMD Rep. 2012. PMID: 23430896 Free PMC article. - Adipocyte size fluctuation, mechano-active lipid droplets and caveolae.
Le Lay S, Briand N, Dugail I. Le Lay S, et al. Adipocyte. 2014 Dec 17;4(2):158-60. doi: 10.4161/21623945.2014.973774. eCollection 2015 Apr-Jun. Adipocyte. 2014. PMID: 26167412 Free PMC article. - The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline.
Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. Brown RJ, et al. J Clin Endocrinol Metab. 2016 Dec;101(12):4500-4511. doi: 10.1210/jc.2016-2466. Epub 2016 Oct 6. J Clin Endocrinol Metab. 2016. PMID: 27710244 Free PMC article. Review. - Novel metabolic disorders in skeletal muscle of Lipodystrophic Bscl2/Seipin deficient mice.
Xu W, Zhou H, Xuan H, Saha P, Wang G, Chen W. Xu W, et al. Mol Cell Endocrinol. 2019 Feb 15;482:1-10. doi: 10.1016/j.mce.2018.12.001. Epub 2018 Dec 4. Mol Cell Endocrinol. 2019. PMID: 30521848 Free PMC article. - Molecular and Cellular Bases of Lipodystrophy Syndromes.
Zammouri J, Vatier C, Capel E, Auclair M, Storey-London C, Bismuth E, Mosbah H, Donadille B, Janmaat S, Fève B, Jéru I, Vigouroux C. Zammouri J, et al. Front Endocrinol (Lausanne). 2022 Jan 3;12:803189. doi: 10.3389/fendo.2021.803189. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046902 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases