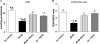NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes - PubMed (original) (raw)
NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes
Nithya Mariappan et al. Cardiovasc Res. 2010.
Abstract
Aims: Inflammatory molecules and their transcription factor, nuclear factor kappa-B (NF-kappaB), are thought to play important roles in diabetes-induced cardiac dysfunction. Here, we investigated the effects of pyrrolidine dithiocarbamate (PDTC), a NF-kappaB inhibitor, in diabetic mice.
Methods and results: Obese db/db mice and heterozygous lean mice (n = 8) were allowed free access to drinking water (control) or water containing PDTC (100 mg/kg) for 20 weeks. Left ventricular (LV) function was measured using echocardiography at baseline and at study end. Mice were sacrificed and LV removed for gene expression, biochemical, immunofluorescence, and mitochondrial assays. LV and mitochondrial reactive oxygen species (ROS), superoxide and peroxynitrite were measured using electron spin resonance spectroscopy. Enhanced NF-kappaB activity in db/db mice was associated with increased oxidative stress as demonstrated by increased ROS, superoxide, and peroxynitrite production, and increased NF-kappaB, gp91phox, and Nox1 expression; PDTC ameliorated these effects. Mitochondrial free radical production and structural damage were higher in the db/db group than in the control, db/db PDTC, and PDTC-treated heterozygous animal groups.
Conclusion: This study demonstrates that NF-kappaB blockade with PDTC mitigates oxidative stress and improves mitochondrial structural integrity directly, through down-regulation of increased oxygen-free radicals, thereby increasing ATP synthesis and thus restoring cardiac function in type II diabetes.
Figures
Figure 1
(A) Mean GSH levels for each experimental group as determined by a kit method. (B) Mean GSH/GSSG ratios for each experimental group. Note the marked decrease in GSH levels and GSH/GSSG ratio for the db/db group and the normalization of these parameters by PDTC treatment. *P < 0.05 vs. control; #P < 0.05 vs. db/db; $P < 0.05 vs. db/db PDTC; @P < 0.05 vs. hz PDTC.
Figure 2
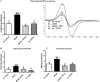
Gene expression levels of (A) gp91phox and (B) Nox1 as determined by real-time RT–PCR and western blotting. Both of these genes were significantly upregulated in db/db LV tissue, and PDTC treatment attenuated expression of these genes. Production levels of (C) superoxide, (D) total ROS, and (E) peroxynitrite as determined by EPR spectroscopy. Levels of superoxide, total ROS, and peroxynitrite production were all increased significantly in db/db LV tissue; these levels were normalized with PDTC treatment. (F) Protein expression levels of 3-nitrotyrosine in LV tissues from all experimental groups. *P < 0.05 vs. control; #P < 0.05 vs. db/db; $P < 0.05 vs. db/db PDTC; @P < 0.05 vs. hz PDTC.
Figure 3
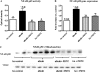
(A and B) Mitochondrial purity as determined by transmission electron microscopy (TEM) and western blot for the mitochondrial markers ANT and VDAC. (C) Results from swelling assay presented in graphical form. (D) Ultrastructural changes of LV tissue and isolated LV mitochondria as examined with TEM; (×40 magnification). Note the disordered appearance of the mitochondria and lack of cristae in db/db animals, and the preservation of appearance with PDTC treatment.
Figure 4
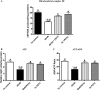
Mitochondrial production rates of (A) ROS, (B) superoxide, and (C) hydrogen peroxide, as determined by EPR, were significantly higher in db/db mice than in any other group. PDTC treatment normalized production rates of ROS, superoxide, and hydrogen peroxide in isolated LV mitochondria. *P < 0.05 vs. control; #P < 0.05 vs. db/db; $P < 0.05 vs. db/db PDTC; @P < 0.05 vs. hz PDTC.
Figure 5
(A) Mitochondrial complex III activity as determined by EPR, (B) ATP production, and (C) ATP/ADP ratios were all decreased in db/db animals when compared with other groups. Treatment with PDTC restored mitochondrial complex III activity and ATP production and improved ATP/ADP ratio. *P < 0.05 vs. control; #P < 0.05 vs. db/db; $P < 0.05 vs. db/db PDTC; @P < 0.05 vs. hz PDTC.
Figure 6
(A) NF-κB p65 activity as determined by ELISA was significantly higher in db/db mice and was attenuated with PDTC treatment. (B) Tissue gene expression and (C) mitochondrial protein expression of NF-κB p50 (as determined by real-time RT–PCR and western blot, respectively) were significantly higher in db/db animals; this expression was attenuated, but not normalized, with PDTC treatment. *P < 0.05 vs. control; #P < 0.05 vs. db/db; $P < 0.05 vs. db/db PDTC; @ < 0.05 vs. hz PDTC.
Similar articles
- Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR.
Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Elks CM, et al. Am J Physiol Renal Physiol. 2009 Feb;296(2):F298-305. doi: 10.1152/ajprenal.90628.2008. Epub 2008 Dec 10. Am J Physiol Renal Physiol. 2009. PMID: 19073636 Free PMC article. - Chronic NF-κB blockade improves renal angiotensin II type 1 receptor functions and reduces blood pressure in Zucker diabetic rats.
Luo H, Wang X, Wang J, Chen C, Wang N, Xu Z, Chen S, Zeng C. Luo H, et al. Cardiovasc Diabetol. 2015 Jun 10;14:76. doi: 10.1186/s12933-015-0239-7. Cardiovasc Diabetol. 2015. PMID: 26055622 Free PMC article. - Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: in vivo demonstration with magnetic resonance imaging.
Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari Z, Zhang C. Zhang H, et al. Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H985-94. doi: 10.1152/ajpheart.00489.2010. Epub 2010 Jul 30. Am J Physiol Heart Circ Physiol. 2010. PMID: 20675566 Free PMC article. - Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension.
Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Kang YM, et al. Cardiovasc Res. 2009 Jun 1;82(3):503-12. doi: 10.1093/cvr/cvp073. Epub 2009 Feb 25. Cardiovasc Res. 2009. PMID: 19246475 Free PMC article. - Myocardial function after gut ischemia/reperfusion: does NF kappaB play a role?
Fischer UM, Radhakrishnan RS, Uray KS, Xue H, Cox CS Jr. Fischer UM, et al. J Surg Res. 2009 Apr;152(2):264-70. doi: 10.1016/j.jss.2008.03.053. Epub 2008 May 5. J Surg Res. 2009. PMID: 18541267
Cited by
- Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy.
Zhang N, Yang Z, Xiang SZ, Jin YG, Wei WY, Bian ZY, Deng W, Tang QZ. Zhang N, et al. Mol Cell Biochem. 2016 Jun;417(1-2):87-96. doi: 10.1007/s11010-016-2716-z. Epub 2016 May 10. Mol Cell Biochem. 2016. PMID: 27160937 - Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling.
Vacek TP, Vacek JC, Tyagi SC. Vacek TP, et al. Arch Physiol Biochem. 2012 Feb;118(1):31-42. doi: 10.3109/13813455.2011.635660. Epub 2011 Dec 19. Arch Physiol Biochem. 2012. PMID: 22181043 Free PMC article. Review. - Tempol treatment normalizes membrane expression of epithelial transport proteins in the kidney of salt-loaded hypertensive diabetic db/db mice.
Dogan YE, Bala N, Chacko KM, Tuna KM, Alli AA. Dogan YE, et al. Am J Transl Res. 2023 Dec 15;15(12):6690-6700. eCollection 2023. Am J Transl Res. 2023. PMID: 38186979 Free PMC article. - Dietary EPA Increases Rat Mortality in Diabetes Mellitus, A Phenomenon Which Is Compensated by Green Tea Extract.
Leger T, He B, Azarnoush K, Jouve C, Rigaudiere JP, Joffre F, Bouvier D, Sapin V, Pereira B, Demaison L. Leger T, et al. Antioxidants (Basel). 2019 Nov 4;8(11):526. doi: 10.3390/antiox8110526. Antioxidants (Basel). 2019. PMID: 31690052 Free PMC article. - Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence?
Correia-Melo C, Hewitt G, Passos JF. Correia-Melo C, et al. Longev Healthspan. 2014 Jan 16;3(1):1. doi: 10.1186/2046-2395-3-1. Longev Healthspan. 2014. PMID: 24472138 Free PMC article.
References
- NIDDK. Bethesda, MD: US Department of Health and Human Services, NIH; 2008. National Diabetes Statistics, 2007.
- AHA. Metabolic Syndrome Statistics Statistical Fact Sheets. Dallas, TX: American Heart Association; 2009.
- Kelishadi R, Gharipour M, Sadri GH, Tavasoli AA, Amani A. Cardiovascular disease risk factors, metabolic syndrome and obesity in an Iranian population. East Mediterr Health J. 2008;14:1070–1079. - PubMed
- Khan KA, Sowers JR. Surgical treatment of the cardiometabolic syndrome and obesity. J Cardiometab Syndr. 2008;3:254–257. - PubMed
- Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1:181–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous