Estrogen action: a historic perspective on the implications of considering alternative approaches - PubMed (original) (raw)
Review
Estrogen action: a historic perspective on the implications of considering alternative approaches
Elwood V Jensen et al. Physiol Behav. 2010.
Abstract
In the 50 years since the initial reports of a cognate estrogen receptor (ER), much has been learned about the diverse effects and mechanisms of estrogens, such as 17beta-estradiol (E(2)). This expert narrative review briefly summarizes perspectives and/or recent work of the authors, who have been addressing different aspects of estrogen action, but take a common approach of using alternative considerations to gain insight into mechanisms with clinical relevance, and inform future studies, regarding estrogen action. Their "Top Ten" favorite alternatives that are discussed herein are as follows. 1 - E(2) has actions by binding to a receptor that do not require its enzymatic conversion. 2 - Using a different strategy for antibody binding could make the estrogen receptor (ER) more discernible. 3 - Blocking ERs, rather than E(2) production, may be a useful strategy for breast cancer therapy. 4 - Secretion of alpha-fetoprotein (AFP), rather than only levels of E(2) and/or progesterone, may influence breast cancer risk. 5 - A peptide derived from the active site of AFP can produce the same benefits of the entire endogenous protein in endocrine cancers. 6 - Differential distribution of ER subtypes in the body and brain may underlie specific effects of estrogens. 7 - ERbeta may be sufficient for the trophic effects of estrogen in the brain, and ERalpha may be the primary target of trophic effects in the body. 8 - ERbeta may play a role in the trophic effects of androgens, and may also be relevant in the periphery. 9 - Downstream of E(2)'s effects at ERbeta, there may be consequences for biosynthesis of progestogens and/or androgens. 10 - Changes in histones and/or other factors, which may be downstream of ERbeta, potentially underlie the divergent effects of E(2) in the brain and peripheral tissues.
2009 Elsevier Inc. All rights reserved.
Figures
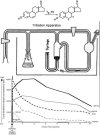
Fig. 1
Top: A cartoon representation of the apparatus that was utilized to tritiate estradiol. Bottom: Graph below demonstrates the amount of radioactivity (dpm; as a measure of tissue sensitivity to estradiol) in various organs (uterus, vagina, liver, kidney, blood, muscle) up to 16 hours after injection of tritiated estradiol. Based upon results reported in [2,3].
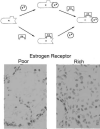
Fig. 2
Top: Schematic of estrogen binding to antibody and receptor. Bottom: Immunocytochemistry examples of how estrogen receptor (ER) antibody was utilized to stain ER expression in mammary tumor tissue, with the sample on the left having low ER immunoreactivity (“ER poor”) and the sample on the right have more ER immunoreactivity (“ER rich”).
Fig. 3
Depiction of typical rates of estrogen receptor (ER) rich or ER poor breast cancer. This information was then used to put patient on endocrine and/or chemotherapy. A better outcome was demonstrated for those who had ER rich breast cancer with endocrine therapy. However, a question remained on how to treat the ~12% of patients who have ER rich tumors, but do not respond to endocrine therapy.
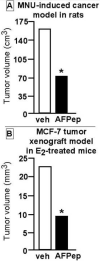
Fig. 4
Tumor volumes following α-fetoptotein (AFP) peptide treatment (AFPep) in two animal models of breast cancer (carcinogen-induced cancer model in rats (A) and MCF-7 tumor xenograft model in mice (B). In both cases, volumes of tumors across groups at the end of the experiment were reduced by administration of AFPep compared to vehicle (* p<0.05). Based upon results reported in [52,53].
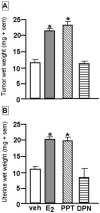
Fig. 5
Tumor (A) and uterine (B) wet weight (mean ± sem) of rats that were administered placebo vehicle, estradiol (E2), or an ERα-SERM (propyl pyrazole triol- PPT), or an ERβ-SERM (diarylpropionitrile- DPN) once a week for 14 weeks. E2 and PPT increased these weights compared to vehicle (* p<0.05).
Fig. 6
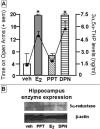
Anxiety behavior in the elevated plus maze (A) of rats administered placebo vehicle, estradiol (E2), or an ERα-SERM (propyl pyrazole triol- PPT), or an ERβ-SERM (diarylpropionitrile- DPN). E2 and DPN increased time spent on the open arms of the elevated plus maze compared to vehicle (* P<0.05). Circles indicate plasma levels of 3α,5α-THP (mean ± sem). Western blot showing representative bands (B) of the 5α-reductase type 1 enzyme, and the loading control β-actin, in the hippocampus of rats treated with vehicle, PPT, E2, or DPN. E2 and DPN increase expression of 5α-reductase in these samples.
Fig. 7
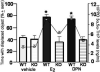
Cognitive performance of adult female mice (genetic knockouts for ERβ- KO; or their wildtype-WT counterparts) administered placebo vehicle, 17β-estradiol (E2), or diarylpropionitrile (DPN). E2 and DPN enhanced performance in this task of WT, but not KO, mice, compared to vehicle administration (* P<0.05). Circles indicate hippocampus levels of 3α,5α-THP in mice. KO mice have lower levels of 3α,5α-THP in the hippocampus than do WT mice after administration of E2 or DPN. Based upon results reported in [111].
Fig. 8
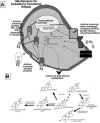
Some of the potential mechanisms, in the cell itself (A) or via metabolism to other neuroactive sterods (B), of estradiol (E2) for its functional effects. E2 can have classic effects to bind to estrogen receptors (ERs), such as ERα and ERβ, and have transcriptional activity. E2 may interact with binding proteins, such as α-foteptrotein (AFP). E2 may also interact with other membrane receptors, such as an ER (which may potentiate intracellular ER function), G-protein coupled receptors (GPCRs), growth factors, and/or neurotransmitter systems, which may involve activation of signal transduction pathways (e.g. adenosine 3′,5′-monophosphate- cAMP, protein kinase A- PKA, phospholipase C- PLC, protein kinase C- PKC, mitogen activating protein kinase- MAPK, extracellular signal-regulated kinase- ERK1/2). E2's functional effects through ERβ may be via altering steroid metabolism of progestogens and androgens.
Similar articles
- Divergent mechanisms for trophic actions of estrogens in the brain and peripheral tissues.
Walf AA, Paris JJ, Rhodes ME, Simpkins JW, Frye CA. Walf AA, et al. Brain Res. 2011 Mar 16;1379:119-36. doi: 10.1016/j.brainres.2010.11.081. Epub 2010 Dec 1. Brain Res. 2011. PMID: 21130078 Free PMC article. Review. - The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems.
Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. Kuiper GG, et al. Front Neuroendocrinol. 1998 Oct;19(4):253-86. doi: 10.1006/frne.1998.0170. Front Neuroendocrinol. 1998. PMID: 9799586 Review. - Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.
Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Katzenellenbogen BS, et al. J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review. - Estrogen receptors: selective ligands, partners, and distinctive pharmacology.
Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. Katzenellenbogen BS, et al. Recent Prog Horm Res. 2000;55:163-93; discussion 194-5. Recent Prog Horm Res. 2000. PMID: 11036937 Review. - Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways.
Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Li X, et al. Mol Cell Biol. 2004 Sep;24(17):7681-94. doi: 10.1128/MCB.24.17.7681-7694.2004. Mol Cell Biol. 2004. PMID: 15314175 Free PMC article.
Cited by
- Estrogen/ER in anti-tumor immunity regulation to tumor cell and tumor microenvironment.
Wang T, Jin J, Qian C, Lou J, Lin J, Xu A, Xia K, Jin L, Liu B, Tao H, Yang Z, Yu W. Wang T, et al. Cancer Cell Int. 2021 Jun 7;21(1):295. doi: 10.1186/s12935-021-02003-w. Cancer Cell Int. 2021. PMID: 34098945 Free PMC article. Review. - Gene transcription regulation by ER at the single cell and allele level.
Stossi F, Rivera Tostado A, Johnson HL, Mistry RM, Mancini MG, Mancini MA. Stossi F, et al. Steroids. 2023 Dec;200:109313. doi: 10.1016/j.steroids.2023.109313. Epub 2023 Sep 25. Steroids. 2023. PMID: 37758052 Free PMC article. - The impact of ERα action on muscle metabolism and insulin sensitivity - Strong enough for a man, made for a woman.
Hevener AL, Zhou Z, Moore TM, Drew BG, Ribas V. Hevener AL, et al. Mol Metab. 2018 Sep;15:20-34. doi: 10.1016/j.molmet.2018.06.013. Epub 2018 Jun 21. Mol Metab. 2018. PMID: 30005878 Free PMC article. Review. - Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex.
Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, Tarakhovsky A, Greengard P. Schaefer A, et al. Neuron. 2009 Dec 10;64(5):678-91. doi: 10.1016/j.neuron.2009.11.019. Neuron. 2009. PMID: 20005824 Free PMC article.
References
- Jensen EV. High point. Breast Cancer Res Treat. 1987;9:77–86. - PubMed
- Jensen EV, Jacobson HI. Fate of steroid estrogens in target tissues. In: Pincus G, Vollmer EP, editors. Biological activities of steroids in relation to cancer. Academic Press; New York: 1960. pp. 161–174.
- Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414.
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH067698-01A2/MH/NIMH NIH HHS/United States
- R01 MH067698/MH/NIMH NIH HHS/United States
- MH06769801/MH/NIMH NIH HHS/United States
- R01 MH067698-03/MH/NIMH NIH HHS/United States
- R01 MH067698-04/MH/NIMH NIH HHS/United States
- R01 MH067698-05/MH/NIMH NIH HHS/United States
- R01 MH067698-05S1/MH/NIMH NIH HHS/United States
- R01 MH067698-02/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources