Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila - PubMed (original) (raw)
Comparative Study
Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila
Chee-Hoe Ng et al. J Neurosci. 2009.
Abstract
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are currently recognized as the most common genetic cause of parkinsonism. Among the large number of LRRK2 mutations identified to date, the G2019S variant is the most common. In Asia, however, another LRRK2 variant, G2385R, appears to occur more frequently. To better understand the contribution of different LRRK2 variants toward disease pathogenesis, we generated transgenic Drosophila over-expressing various human LRRK2 alleles, including wild type, G2019S, Y1699C, and G2385R LRRK2. We found that transgenic flies harboring G2019S, Y1699C, or G2385R LRRK2 variant, but not the wild-type protein, exhibit late-onset loss of dopaminergic (DA) neurons in selected clusters that is accompanied by locomotion deficits. Furthermore, LRRK2 mutant flies also display reduced lifespan and increased sensitivity to rotenone, a mitochondrial complex I inhibitor. Importantly, coexpression of human parkin in LRRK2 G2019S-expressing flies provides significant protection against DA neurodegeneration that occurs with age or in response to rotenone. Together, our results suggest a potential link between LRRK2, parkin, and mitochondria in the pathogenesis of LRRK2-related parkinsonism.
Figures
Figure 1.
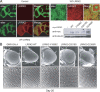
Expression of LRRK2 transgenes in Drosophila neither affects its overall brain architecture nor triggers obvious eye abnormalities. A, Left, Anti-elav (green) and anti-LRRK2 (red) immunostaining of whole-mount adult brains derived from 2-d-old control or transgenic flies expressing wild-type human LRRK2, as indicated. Right, Enlarged images (and inset) show the localization of elav and LRRK2 signals to the nucleus and cytoplasm, respectively, in the brain of a wild-type LRRK2-expressing fly. An anti-myc immunoblot of brain lysates prepared from 2-d-old control or transgenic flies expressing various LRRK2 species is shown below (genotype: elav-Gal4/+ or elav–Gal4–hLRRK2). B, SEM eye images of 20-d-old GMR-Gal4/+ or GMR–Gal4–hLRRK adult flies. No retinal degeneration was observed in all flies examined.
Figure 2.
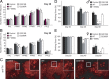
Expression of LRRK2 mutants in flies promotes DA neurodegeneration and concomitant locomotion deficits. A, B, Bar graph showing the number of TH-positive DA neurons in different clusters of various fly species at 20 or 60 d after eclosion, as indicated (n = 10). C, Representative confocal microscopy images showing TH-positive (red) DA neurons in the PPM1 cluster (boxed) of 60-d-old control or LRRK2-expressing flies. Inset, Higher magnification of boxed regions. D, E, Bar graph showing the percentage of various male (D) and female (E) flies at different days after eclosion that reached the top of assay column after 1 min (n = 20) (genotype: ddc–Gal4/+ or ddc–Gal4–hLRRK2).
Figure 3.
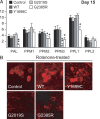
Exposure to rotenone accelerates DA degeneration in LRRK2 G2019 and G2385R mutant flies. A, Bar graph showing the number of TH-positive DA neurons in different clusters of various fly species at 15 d after rotenone treatment (n = 15). B, Representative confocal microscopy images showing TH-positive (red) DA neurons in the PPM3 cluster of various, rotenone-treated fly species, as indicated (genotype: ddc–Gal4/+ or ddc–Gal4–hLRRK2).
Figure 4.
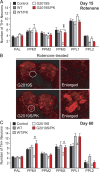
Parkin coexpression mitigates DA degeneration in LRRK2 G2019S-expressing flies in the presence or absence of rotenone. A, Bar graph showing the number of TH-positive DA neurons in different clusters of various fly species at 15 d after rotenone treatment (n = 15). B, Representative confocal microscopy images showing TH-positive (red) DA neurons in whole-mount adult brains derived from rotenone-treated flies expressing either LRRK2 G2019S alone or in the presence of parkin coexpression. Right, PPM3 cluster (circled) are shown at higher magnification. C, Bar graph showing the number of TH-positive DA neurons in different clusters of the various fly species at 60 d after eclosion, as indicated (n = 8) (genotype: ddc–Gal4/+ or ddc–Gal4–hLRRK2 or ddci-Gal4–hLRRK2;ddc–Gal4–hparkin).
Comment in
- Unraveling LRRK2 pathogenesis: common pathways for complex genes?
Deas E, Dunn L. Deas E, et al. J Neurosci. 2010 Feb 3;30(5):1577-9. doi: 10.1523/JNEUROSCI.5531-09.2010. J Neurosci. 2010. PMID: 20130167 Free PMC article. No abstract available.
Similar articles
- Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities.
Wang C, Lu R, Ouyang X, Ho MW, Chia W, Yu F, Lim KL. Wang C, et al. J Neurosci. 2007 Aug 8;27(32):8563-70. doi: 10.1523/JNEUROSCI.0218-07.2007. J Neurosci. 2007. PMID: 17687034 Free PMC article. - A Drosophila model for LRRK2-linked parkinsonism.
Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, Ross CA, Montell C, Smith WW. Liu Z, et al. Proc Natl Acad Sci U S A. 2008 Feb 19;105(7):2693-8. doi: 10.1073/pnas.0708452105. Epub 2008 Feb 7. Proc Natl Acad Sci U S A. 2008. PMID: 18258746 Free PMC article. - Thiol peroxidases ameliorate LRRK2 mutant-induced mitochondrial and dopaminergic neuronal degeneration in Drosophila.
Angeles DC, Ho P, Chua LL, Wang C, Yap YW, Ng C, Zhou Zd, Lim KL, Wszolek ZK, Wang HY, Tan EK. Angeles DC, et al. Hum Mol Genet. 2014 Jun 15;23(12):3157-65. doi: 10.1093/hmg/ddu026. Epub 2014 Jan 23. Hum Mol Genet. 2014. PMID: 24459295 Free PMC article. - The LRRK2 G2019S mutation as the cause of Parkinson's disease in Ashkenazi Jews.
Thaler A, Ash E, Gan-Or Z, Orr-Urtreger A, Giladi N. Thaler A, et al. J Neural Transm (Vienna). 2009 Nov;116(11):1473-82. doi: 10.1007/s00702-009-0303-0. J Neural Transm (Vienna). 2009. PMID: 19756366 Review. - Clinical features of LRRK2 parkinsonism.
Haugarvoll K, Wszolek ZK. Haugarvoll K, et al. Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S205-8. doi: 10.1016/S1353-8020(09)70815-6. Parkinsonism Relat Disord. 2009. PMID: 20082991 Review.
Cited by
- Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice.
Yue M, Hinkle KM, Davies P, Trushina E, Fiesel FC, Christenson TA, Schroeder AS, Zhang L, Bowles E, Behrouz B, Lincoln SJ, Beevers JE, Milnerwood AJ, Kurti A, McLean PJ, Fryer JD, Springer W, Dickson DW, Farrer MJ, Melrose HL. Yue M, et al. Neurobiol Dis. 2015 Jun;78:172-95. doi: 10.1016/j.nbd.2015.02.031. Epub 2015 Mar 31. Neurobiol Dis. 2015. PMID: 25836420 Free PMC article. - Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis.
Weindel CG, Martinez EL, Zhao X, Mabry CJ, Bell SL, Vail KJ, Coleman AK, VanPortfliet JJ, Zhao B, Wagner AR, Azam S, Scott HM, Li P, West AP, Karpac J, Patrick KL, Watson RO. Weindel CG, et al. Cell. 2022 Aug 18;185(17):3214-3231.e23. doi: 10.1016/j.cell.2022.06.038. Epub 2022 Jul 30. Cell. 2022. PMID: 35907404 Free PMC article. - Lessons on Differential Neuronal-Death-Vulnerability from Familial Cases of Parkinson's and Alzheimer's Diseases.
Franco R, Navarro G, Martínez-Pinilla E. Franco R, et al. Int J Mol Sci. 2019 Jul 4;20(13):3297. doi: 10.3390/ijms20133297. Int J Mol Sci. 2019. PMID: 31277513 Free PMC article. Review. - Dysregulated LRRK2 signaling in response to endoplasmic reticulum stress leads to dopaminergic neuron degeneration in C. elegans.
Yuan Y, Cao P, Smith MA, Kramp K, Huang Y, Hisamoto N, Matsumoto K, Hatzoglou M, Jin H, Feng Z. Yuan Y, et al. PLoS One. 2011;6(8):e22354. doi: 10.1371/journal.pone.0022354. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21857923 Free PMC article. - Decreased Sirtuin Deacetylase Activity in LRRK2 G2019S iPSC-Derived Dopaminergic Neurons.
Schwab AJ, Sison SL, Meade MR, Broniowska KA, Corbett JA, Ebert AD. Schwab AJ, et al. Stem Cell Reports. 2017 Dec 12;9(6):1839-1852. doi: 10.1016/j.stemcr.2017.10.010. Epub 2017 Nov 9. Stem Cell Reports. 2017. PMID: 29129681 Free PMC article.
References
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. - PubMed
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases