Asymptomatic deer excrete infectious prions in faeces - PubMed (original) (raw)
. 2009 Sep 24;461(7263):529-32.
doi: 10.1038/nature08289. Epub 2009 Sep 9.
Affiliations
- PMID: 19741608
- PMCID: PMC3186440
- DOI: 10.1038/nature08289
Asymptomatic deer excrete infectious prions in faeces
Gültekin Tamgüney et al. Nature. 2009.
Erratum in
- Nature. 2010 Jul 29;466(7306):652. Dosage error in article text
Abstract
Infectious prion diseases-scrapie of sheep and chronic wasting disease (CWD) of several species in the deer family-are transmitted naturally within affected host populations. Although several possible sources of contagion have been identified in excretions and secretions from symptomatic animals, the biological importance of these sources in sustaining epidemics remains unclear. Here we show that asymptomatic CWD-infected mule deer (Odocoileus hemionus) excrete CWD prions in their faeces long before they develop clinical signs of prion disease. Intracerebral inoculation of irradiated deer faeces into transgenic mice overexpressing cervid prion protein (PrP) revealed infectivity in 14 of 15 faecal samples collected from five deer at 7-11 months before the onset of neurological disease. Although prion concentrations in deer faeces were considerably lower than in brain tissue from the same deer collected at the end of the disease, the estimated total infectious dose excreted in faeces by an infected deer over the disease course may approximate the total contained in a brain. Prolonged faecal prion excretion by infected deer provides a plausible natural mechanism that might explain the high incidence and efficient horizontal transmission of CWD within deer herds, as well as prion transmission among other susceptible cervids.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
Kaplan-Meier plots indicating incubation times in Tg(ElkPrP) mice after i.c. inoculation with 1% (wt/vol) brain homogenate from mule deer (ID numbers indicated) that developed CWD in 16–20 months after oral infection with CWD prions.
Fig. 2
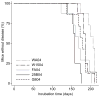
Feces from 5 mule deer (WA04, circle; W1504, square; FA04, triangle; 25B04, inverted triangle; G804, diamond) were sampled before and at 4 time points following oral infection; the last collection was taken when the deer developed signs of CWD. Irradiated fecal homogenates were i.c. inoculated in Tg(ElkPrP) mice, some of which developed prion disease between 153–470 dpi when inoculated with fecal preparations collected from deer >4 months after their oral infection. Feces collected from deer before (data not shown) or at 3–4 months after oral infection did not transmit disease to Tg(ElkPrP) mice.
Fig. 3
Western blots of brain homogenates of Tg(ElkPrP) mice inoculated i.c. with feces (A) or brain homogenates (B) from mule deer. (A) Mice inoculated with mule deer feces collected before the deer were orally infected with CWD prions were sacrificed at 481 dpi and showed no PK-resistant PrPSc in their brains. In contrast, some mice inoculated with feces from CWD-infected mule deer collected at >4 months after oral infection had PrPSc in their brains. (B) Inoculation with brain homogenates from CWD- infected deer resulted in prion disease and PrPSc in the brains of ill Tg(ElkPrP) mice. Samples were undigested (−) or digested with proteinase K (+). Molecular masses of protein standards are shown in kilodaltons (kDa).
Fig. 4
Neuropathology of brain sections from Tg(ElkPrP) mice inoculated with fecal (d–i) or brain (j–l) homogenates of mule deer. Sections show vacuolation (left), PrPSc deposition (middle), and astrocytic gliosis (right). Uninoculated, 658-day-old, control mice showed no vacuolation (a), no PrPSc deposits (b), and mild, age-related gliosis (c). Mice inoculated with feces from uninfected deer remained healthy without vacuolation (d) or PrPSc deposits (e), and showed mild, age-related gliosis at 503 dpi (f). Mice inoculated with feces from infected deer developed neurologic symptoms in 153–438 days, showed vacuolation (g), PrPSc deposits (h), and severe gliosis (i) similar to mice inoculated with brain homogenates of deer with CWD (j–l). Bar, 25 μm.
Similar articles
- Faecal CWD prion excretion and inflammation.
Di Guardo G, Marruchella G. Di Guardo G, et al. New Microbiol. 2010 Apr;33(2):183-4. New Microbiol. 2010. PMID: 20518283 - Temporal patterns of chronic wasting disease prion excretion in three cervid species.
Plummer IH, Wright SD, Johnson CJ, Pedersen JA, Samuel MD. Plummer IH, et al. J Gen Virol. 2017 Jul;98(7):1932-1942. doi: 10.1099/jgv.0.000845. Epub 2017 Jul 15. J Gen Virol. 2017. PMID: 28708047 - Longitudinal Detection of Prion Shedding in Saliva and Urine by Chronic Wasting Disease-Infected Deer by Real-Time Quaking-Induced Conversion.
Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. Henderson DM, et al. J Virol. 2015 Sep;89(18):9338-47. doi: 10.1128/JVI.01118-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136567 Free PMC article. - Chronic wasting disease of cervids.
Miller MW, Williams ES. Miller MW, et al. Curr Top Microbiol Immunol. 2004;284:193-214. doi: 10.1007/978-3-662-08441-0_8. Curr Top Microbiol Immunol. 2004. PMID: 15148993 Review. - Chronic wasting disease in Europe: new strains on the horizon.
Tranulis MA, Gavier-Widén D, Våge J, Nöremark M, Korpenfelt SL, Hautaniemi M, Pirisinu L, Nonno R, Benestad SL. Tranulis MA, et al. Acta Vet Scand. 2021 Nov 25;63(1):48. doi: 10.1186/s13028-021-00606-x. Acta Vet Scand. 2021. PMID: 34823556 Free PMC article. Review.
Cited by
- Prion Seeding Activity in Plant Tissues Detected by RT-QuIC.
Burgener K, Lichtenberg SS, Walsh DP, Inzalaco HN, Lomax A, Pedersen JA. Burgener K, et al. Pathogens. 2024 May 26;13(6):452. doi: 10.3390/pathogens13060452. Pathogens. 2024. PMID: 38921750 Free PMC article. - Chimeric elk/mouse prion proteins in transgenic mice.
Tamgüney G, Giles K, Oehler A, Johnson NL, DeArmond SJ, Prusiner SB. Tamgüney G, et al. J Gen Virol. 2013 Feb;94(Pt 2):443-452. doi: 10.1099/vir.0.045989-0. Epub 2012 Oct 24. J Gen Virol. 2013. PMID: 23100369 Free PMC article. - Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America.
Uehlinger FD, Johnston AC, Bollinger TK, Waldner CL. Uehlinger FD, et al. BMC Vet Res. 2016 Aug 22;12(1):173. doi: 10.1186/s12917-016-0804-7. BMC Vet Res. 2016. PMID: 27549119 Free PMC article. Review. - Age structuring and spatial heterogeneity in prion protein gene (PRNP) polymorphism in white-tailed deer.
Chafin TK, Douglas MR, Martin BT, Zbinden ZD, Middaugh CR, Ballard JR, Gray MC, Don White Jr, Douglas ME. Chafin TK, et al. Prion. 2020 Dec;14(1):238-248. doi: 10.1080/19336896.2020.1832947. Prion. 2020. PMID: 33078661 Free PMC article. - Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein.
Sandberg MK, Al-Doujaily H, Sigurdson CJ, Glatzel M, O'Malley C, Powell C, Asante EA, Linehan JM, Brandner S, Wadsworth JD, Collinge J. Sandberg MK, et al. J Gen Virol. 2010 Oct;91(Pt 10):2651-7. doi: 10.1099/vir.0.024380-0. Epub 2010 Jul 7. J Gen Virol. 2010. PMID: 20610667 Free PMC article.
References
- Prusiner SB. Prions. In: Knipe DM, et al., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 3059–3092.
- Detwiler LA, Baylis M. The epidemiology of scrapie. Rev Sci Tech. 2003;22:121–143. - PubMed
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. - PubMed
- Williams ES. Chronic wasting disease. Vet Pathol. 2005;42:530–549. - PubMed
- Mathiason CK, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials