Crohn's disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly - PubMed (original) (raw)
Crohn's disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly
Pierre Lapaquette et al. Cell Microbiol. 2010 Jan.
Abstract
Ileal lesions in Crohn's disease (CD) patients are colonized by pathogenic adherent-invasive Escherichia coli (AIEC) able to invade and to replicate within intestinal epithelial cells. Recent genome-wide association studies have highlighted the autophagy pathway as being associated with CD risk. In the present study we investigated whether defects in autophagy enhance replication of commensal and pathogenic Escherichia coli and CD-associated AIEC. We show that functional autophagy limits intracellular AIEC replication and that a subpopulation of the intracellular bacteria is located within LC3-positive autophagosomes. In IRGM and ATG16L1 deficient cells intracellular AIEC LF82 bacteria have enhanced replication. Surprisingly autophagy deficiency did not interfere with the ability of intracellular bacteria to survive and/or replicate for any other E. coli strains tested, including non-pathogenic, environmental, commensal, or pathogenic strains involved in gastro enteritis. Together these findings demonstrate a central role for autophagy restraining Adherent-Invasive E. coli strains associated with ileal CD. AIEC infection in patients with polymorphisms in autophagy genes may have a significant impact on the outcome of intestinal inflammation.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
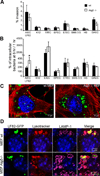
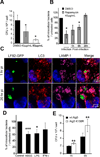
Figure 1. Autophagy restricts the intracellular replication of AIEC LF82 strain but not that of commensal or pathogenic E coli strains
Experiments were performed with wild-type mouse embryonic fibroblasts (black rectangle) and with autophagy deficient atg5−/− MEFs (white rectangle). Infections were performed with the non pathogenic E. coli K-12 strain MG1655, the environmental E. coli strain SMS 3.5, the commensal E. coli strain HS, enteropathogenic E. coli (EPEC) strain E2348/69 (D), the diffusely adhering E. coli (DAEC) strain C1845, the enterotoxigenic E. coli (ETEC) strain H10407, the enteroinvasive E. coli (EIEC) strain E12860/0, and the CD-associated adherent-invasive E. coli (AIEC) strain LF82. The numbers of intracellular bacteria were determined at various times post-infection ranging from 1 h to 6 h. Results are expressed as percent invasion corresponding to mean percentage of the inoculum retrieved as intracellular bacteria after 1 h of gentamycin treatment following 1 h of infection (A) or as percentage of intracellular bacteria after 6 h post-infection relative to that obtained at 1 h after gentamycin exposure, taken as 100% (B). Confocal microscopic examinations of AIEC LF82-GFP infected wt MEFs and autophagy deficient atg5−/− MEFs after 6 h post-infection (C), with bacteria expressing GFP in green, actin in red and nuclei in blue. Representative confocal micrographs of LAMP-1 (purple) and lysotracker (red) labelling of AIEC-LF82 infected wt MEFs and Atg5−/− MEFs at 6 h post infection (D).
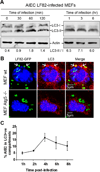
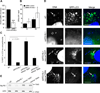
Figure 2. AIEC LF82 bacteria induced autophagy in MEF and were located within autophagosomes
A. Immunoblot analysis using antibodies against LC3 and actin to analyze the LC3 II/LC3 I ratio at early times of infection or post-infection with AIEC LF82. B. Confocal microscopic examinations of AIEC LF82-GFP infected wt MEFs and atg5−/− MEFs at 6 h post-infection to analyze LC3 colocalisation using monoclonal antibody to LC3 (red). Nuclei are in blue. C. Percentage of LC3 positive vacuole containing LF82-GFP bacteria determined by confocal microscopy at various times post-infection. Data shown represent means +/− SEM of three independent experiments, counting 50 cells per experiment.
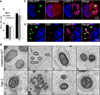
Figure 3. AIEC LF82 bacteria interact with autophagy in epithelial cell lines
A. Intracellular replication of AIEC LF82 bacteria at 1 h and 6 h post-infection within HeLa, Hep-2 and Intestine-407 epithelial cells. Results are expressed as mean numbers +/− SEM of colony forming units (CFU) per well. Each point is the mean of at least three separate experiments. B. Ultrastructural analysis of Intestine-407 and Hep-2 epithelial cells infected with AIEC strain LF82 for 6 h by transmission electron microscopy showing various intracellular compartments for intracellular LF82 bacteria: single bacteria in monolayer membrane vacuoles (1 and 5), several bacteria in a single membrane vacuole (2 and 6), bacteria in damaged vacuole (3 and 7) and bacteria in a multilamellar membrane vacuole also containing sequestered cytoplasm (4 and 8). C. Confocal microscopic examinations of AIEC LF82 GFP-infected Hela cells at 6 h post-infection labelled with lysotracker (red) and for LAMP-1 (purple) to visualize the vacuoles. D. Confocal analysis after permeabilization of plasma membrane with digitonin and labelling of free cytosolic AIEC LF82 with O83 antibodies (red) and of vacuoles with LAMP-1 antibodies (purple).
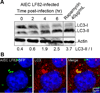
Figure 4. AIEC LF82 infection induces autophagy in epithelial cell lines
A. Immunoblot analysis using antibodies against LC3 B or actin to analyze the LC3 II/LC3 I ratio at various times post-infection with AIEC LF82. Induction of autophagy was performed with rapamycin treatment (40µg/ml for 2 h) as a positive control for LC3 II shift. B. Confocal microscopic examinations of AIEC LF82-GFP (green) infected HeLa at 6 h post-infection to analyze LC3 colocalisation using monoclonal antibody to LC3 (red). Nuclei are in blue.
Figure 5. Pharmacological- and physiological-induced autophagy can restrict the replication of intracellular AIEC LF82 bacteria
A. Rapamycin dose-dependent decrease in the numbers of intracellular AIEC LF82 bacteria. Cells were treated concomitantly with bacterial infection. Results are expressed as mean numbers +/− SEM of colony forming units (CFU) per well at 1 h post-infection. Each point is the mean of at least three separate experiments. B. Comparison of early or late induced autophagy by rapamycin treatment on the ability of AIEC LF82 bacteria to survive and/or to replicate intracellularly. Rapamycin was added during the 3 h infection, or at 1, 6 or 20 h post-infection. Results are expressed as the number of intracellular bacteria in treated cells relative to that obtained in untreated cells, taken as 100%. C. Confocal microscopic examinations of rapamycin treatment of Hela cells infected with AIEC LF82-GFP at 1 h and 20 h post-infection labelled with LC3 (red) and for LAMP-1 (purple). D. Effects of induced autophagy in HeLa cells by starvation or by LPS (100 ng/mL) and IFN-γ(1000U/mL) stimulations on the numbers of intracellular AIEC LF82 bacteria. Results are expressed as the number of intracellular bacteria in treated cells relative to that obtained in untreated cells, taken as 100%. E. Expression of the Atg5_K130R_ dominant negative in HeLa cells leads to increased numbers of intracellular AIEC LF82 bacteria. HeLa cells transfected with plasmid pEGFP-Atg5 encoding wild-type Atg5 (black bars) or with pEGFP-Atg5K130R encoding the Atg5_K130R_ dominant negative (white bars) were infected for 3 h. Results are expressed as mean numbers +/− SEM of colony forming units (CFU) per well. Each point is the mean of at least three separate experiments.
Figure 6. Intracellular AIEC LF82 bacteria replicate within cells impaired in ATG16L1 expression
The siRNA experiments against endogeneous ATG16L1 transcripts were performed in HeLa cells transfected with ATG16L1 specific siRNA oligonucleotides. A. Endogenous ATG16L1 mRNA knockdown by specific siRNA in HeLa cells after 48 h of transfection was assessed by real-time quantitative RT-PCR normalized to GAPDH, compared with control duplexes. RT-PCR was performed in triplicate. Results represent independent experiments. B. ATG16L1 knockdown increases the ability of the AIEC LF82 bacteria to replicate intracellularly. HeLa cells transfected with control (black bar) or ATG16L1 (white bar) -directed siRNA oligos were infected for 3 h. Results are expressed as mean numbers +/− SEM of colony forming units (CFU) per well. Each point is the mean of at least three separate experiments. C. ATG16L1 knockdown and allele-specific rescue reveals compromised autophagy of AIEC mediated by the CD-associated *300A allele. HeLa-GFP-LC3 cells were transfected with control (siC) or ATG16L1-targeting siRNA duplexes, along with empty plasmid (siATG16L1), or allele-specific rescue constructs prior to infection with AIEC. After three hours of infection, cells were fixed and stained for microscopic analysis. Data shown represent means +/− SEM of three independent experiments, counting 50 cells per experiment. Significance was determined using two-tailed Student’s T-tests with Bonferroni correction for multiple comparisons. D. Confocal microscopic examination of colocalization of intracellular AIEC LF82 bacteria with GFP-LC3 in HeLa-GFP-LC3 cells transfected with control or ATG16L1-targeting siRNA duplexes, along with empty plasmid, or allele-specific rescue constructs prior to infection with AIEC. E. ATG16L1 knockdown and rescue results in similar levels of allele-specific expression. Expression of both rescue constructs was equal, as determined by Western blotting with ATG16L1-specific antibodies, using actin as a loading control.
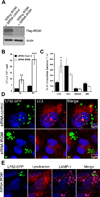
Figure 7. IRGM, a CD-associated genetic marker, controls the number of intracellular AIEC LF82 bacteria within epithelial cells
The siRNA experiments against endogeneous IRGM transcripts were performed in HeLa cells cotransfected with the Flag-tagged IRGM expression plasmid pCMV-3xFlag and with IRGM specific siRNA oligonucleotides. A. Immunoblot using anti-Flag antibody to analyze IRGM expression. B. IRGM knockdown increases the ability of the AIEC LF82 bacteria to replicate intracellularly. HeLa cells transfected with control (black bar) or IRGM (white bar) -directed siRNA oligos were infected for 3 h. Results are expressed as mean numbers +/− SEM of colony forming units (CFU) per well. Each point is the mean of at least three separate experiments. C. IRGM knockdown does not modify the survival/replication of non pathogenic E. coli. Results are expressed as the number of intracellular bacteria at 6 h post-infection relative to that obtained at 1 h post-infection, taken as 100%. D. Confocal microscopic examination reveals an increased number of intracellular AIEC LF82 bacteria in IRGM knockdown cells. Control siRNA-treated (siControl) and IRGM siRNA-treated (siRNA IRGM) cells were infected with AIEC LF82-GFP bacteria and imaged by confocal microscopy. Control cells show intracellular GFP-bacteria (green) as small clusters enclosed in LC3 positive (red) vacuoles. E. Confocal microscopic examinations of IRGM siRNA-treated Hela cells infected with AIEC LF82 GFP at 6 h post-infection labelled with lysotracker (red) and for LAMP-1 (purple) to visualize the vacuoles.
Similar articles
- Abnormalities in the handling of intracellular bacteria in Crohn's disease.
Lapaquette P, Darfeuille-Michaud A. Lapaquette P, et al. J Clin Gastroenterol. 2010 Sep;44 Suppl 1:S26-9. doi: 10.1097/MCG.0b013e3181dd4fa5. J Clin Gastroenterol. 2010. PMID: 20616747 - Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response.
Lapaquette P, Bringer MA, Darfeuille-Michaud A. Lapaquette P, et al. Cell Microbiol. 2012 Jun;14(6):791-807. doi: 10.1111/j.1462-5822.2012.01768.x. Epub 2012 Mar 1. Cell Microbiol. 2012. PMID: 22309232 - Exosomes transfer miRNAs from cell-to-cell to inhibit autophagy during infection with Crohn's disease-associated adherent-invasive E. coli.
Larabi A, Dalmasso G, Delmas J, Barnich N, Nguyen HTT. Larabi A, et al. Gut Microbes. 2020 Nov 1;11(6):1677-1694. doi: 10.1080/19490976.2020.1771985. Epub 2020 Jun 25. Gut Microbes. 2020. PMID: 32583714 Free PMC article. - Pathogenesis of adherent-invasive Escherichia coli.
Smith EJ, Thompson AP, O'Driscoll A, Clarke DJ. Smith EJ, et al. Future Microbiol. 2013 Oct;8(10):1289-300. doi: 10.2217/fmb.13.94. Future Microbiol. 2013. PMID: 24059919 Review. - Adherent-Invasive E. coli: Update on the Lifestyle of a Troublemaker in Crohn's Disease.
Chervy M, Barnich N, Denizot J. Chervy M, et al. Int J Mol Sci. 2020 May 25;21(10):3734. doi: 10.3390/ijms21103734. Int J Mol Sci. 2020. PMID: 32466328 Free PMC article. Review.
Cited by
- MicroRNA signatures in the pathogenesis and therapy of inflammatory bowel disease.
Ramadan YN, Kamel AM, Medhat MA, Hetta HF. Ramadan YN, et al. Clin Exp Med. 2024 Sep 11;24(1):217. doi: 10.1007/s10238-024-01476-z. Clin Exp Med. 2024. PMID: 39259390 Free PMC article. Review. - Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine.
Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, Conway T. Maltby R, et al. PLoS One. 2013;8(1):e53957. doi: 10.1371/journal.pone.0053957. Epub 2013 Jan 17. PLoS One. 2013. PMID: 23349773 Free PMC article. - Pathogenesis of Crohn's disease: Bug or no bug.
Bosca-Watts MM, Tosca J, Anton R, Mora M, Minguez M, Mora F. Bosca-Watts MM, et al. World J Gastrointest Pathophysiol. 2015 Feb 15;6(1):1-12. doi: 10.4291/wjgp.v6.i1.1. World J Gastrointest Pathophysiol. 2015. PMID: 25685606 Free PMC article. Review. - Autophagy and Digestive Disorders: Advances in Understanding and Therapeutic Approaches.
Thein W, Po WW, Choi WS, Sohn UD. Thein W, et al. Biomol Ther (Seoul). 2021 Jul 1;29(4):353-364. doi: 10.4062/biomolther.2021.086. Biomol Ther (Seoul). 2021. PMID: 34127572 Free PMC article. Review. - The Role of ATG16 in Autophagy and The Ubiquitin Proteasome System.
Xiong Q, Li W, Li P, Yang M, Wu C, Eichinger L. Xiong Q, et al. Cells. 2018 Dec 20;8(1):2. doi: 10.3390/cells8010002. Cells. 2018. PMID: 30577509 Free PMC article. Review.
References
- Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. Isme J. 2007;1:403–418. - PubMed
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. - PubMed
- Birmingham CL, Canadien V, Gouin E, Troy EB, Yoshimori T, Cossart P, et al. Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy. 2007;3:442–451. - PubMed
- Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn’s disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2001;39:1272–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI062773/AI/NIAID NIH HHS/United States
- DK043351/DK/NIDDK NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- DK83756/DK/NIDDK NIH HHS/United States
- P30 DK040561-14/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical