Signaling effect of amyloid-beta(42) on the processing of AbetaPP - PubMed (original) (raw)
Review
Signaling effect of amyloid-beta(42) on the processing of AbetaPP
Massimo Tabaton et al. Exp Neurol. 2010 Jan.
Abstract
The effects of amyloid-beta are extremely complex. Current work in the field of Alzheimer disease is focusing on discerning the impact between the physiological signaling effects of soluble low molecular weight amyloid-beta species and the more global cellular damage that could derive from highly concentrated and/or aggregated amyloid. Being able to dissect the specific signaling events, to understand how soluble amyloid-beta induces its own production by up-regulating BACE1 expression, could lead to new tools to interrupt the distinctive feedback cycle with potential therapeutic consequences. Here we describe a positive loop that exists between the secretases that are responsible for the generation of the amyloid-beta component of Alzheimer disease. According to our hypothesis, in familial Alzheimer disease, the primary overproduction of amyloid-beta can induce BACE1 transcription and drive a further increase of amyloid-beta precursor protein processing and resultant amyloid-beta production. In sporadic Alzheimer disease, many factors, among them oxidative stress and inflammation, with consequent induction of presenilins and BACE1, would activate a loop and proceed with the generation of amyloid-beta and its signaling role onto BACE1 transcription. This concept of a signaling effect by and feedback on the amyloid-beta precursor protein will likely shed light on how amyloid-beta generation, oxidative stress, and secretase functions are intimately related in sporadic Alzheimer disease.
Figures
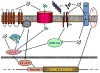
Figure 1. Schematic of the tentative model of Aβ induced activation of BACE1 transcription
Soluble Aβ (blue dots) could activate G-coupled receptors or ionic (Ca++) channels leading to increase Ca++ entry. The same effect could be achieved intracellularly (green dots) via interaction with the ER, liberating Ca++. This could lead to JNK activation and finally AP1-mediated transcription of BACE1. Inhibition of IRs and Akt pathway by Aβ could liberate the action of JNK on AP1, achieving the same effect. Increased production of BACE1 completes the cycle, enhancing the processing of AβPP (red circle) to form more Aβ. Oligomers and fibrils (aggregated blue dots) are formed as a consequence of enhanced amyloidogenesis, but it is not clear if they have a role in this signalling. Blue dots: Aβ; green dots: Ca++; arrows: stimulation; blunt arrows: inhibition; IRs: insulin receptors; G: G-proteins and G-coupled receptors; β and γ: β-and γ-secretase cleavages; ER: endoplasmic reticulum.
Comment in
- Beyond the signaling effect role of amyloid-ß42 on the processing of APP, and its clinical implications.
Lahiri DK, Maloney B. Lahiri DK, et al. Exp Neurol. 2010 Sep;225(1):51-4. doi: 10.1016/j.expneurol.2010.04.018. Epub 2010 May 5. Exp Neurol. 2010. PMID: 20451519 Free PMC article. Review.
Similar articles
- Alternative Selection of β-Site APP-Cleaving Enzyme 1 (BACE1) Cleavage Sites in Amyloid β-Protein Precursor (APP) Harboring Protective and Pathogenic Mutations within the Aβ Sequence.
Kimura A, Hata S, Suzuki T. Kimura A, et al. J Biol Chem. 2016 Nov 11;291(46):24041-24053. doi: 10.1074/jbc.M116.744722. Epub 2016 Sep 29. J Biol Chem. 2016. PMID: 27687728 Free PMC article. - Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.
Deng Y, Wang Z, Wang R, Zhang X, Zhang S, Wu Y, Staufenbiel M, Cai F, Song W. Deng Y, et al. Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065 - Amyloid-β₄₂ activates the expression of BACE1 through the JNK pathway.
Guglielmotto M, Monteleone D, Giliberto L, Fornaro M, Borghi R, Tamagno E, Tabaton M. Guglielmotto M, et al. J Alzheimers Dis. 2011;27(4):871-83. doi: 10.3233/JAD-2011-110884. J Alzheimers Dis. 2011. PMID: 21897006 - Amyloid-β production: major link between oxidative stress and BACE1.
Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Tamagno E, et al. Neurotox Res. 2012 Oct;22(3):208-19. doi: 10.1007/s12640-011-9283-6. Epub 2011 Oct 15. Neurotox Res. 2012. PMID: 22002808 Review. - The β-Secretase Enzyme BACE1: A Biochemical Enigma for Alzheimer's Disease.
Shah H, Patel A, Parikh V, Nagani A, Bhimani B, Shah U, Bambharoliya T. Shah H, et al. CNS Neurol Disord Drug Targets. 2020;19(3):184-194. doi: 10.2174/1871527319666200526144141. CNS Neurol Disord Drug Targets. 2020. PMID: 32452328 Review.
Cited by
- Oxidative DNA Damage Signalling in Neural Stem Cells in Alzheimer's Disease.
Kieroń M, Żekanowski C, Falk A, Wężyk M. Kieroń M, et al. Oxid Med Cell Longev. 2019 Nov 13;2019:2149812. doi: 10.1155/2019/2149812. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31814869 Free PMC article. Review. - Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer's disease and not in other tauopathies.
Pires G, McElligott S, Drusinsky S, Halliday G, Potier MC, Wisniewski T, Drummond E. Pires G, et al. Acta Neuropathol Commun. 2019 Dec 3;7(1):195. doi: 10.1186/s40478-019-0848-6. Acta Neuropathol Commun. 2019. PMID: 31796108 Free PMC article. - Insight into the Molecular Imaging of Alzheimer's Disease.
Arora A, Bhagat N. Arora A, et al. Int J Biomed Imaging. 2016;2016:7462014. doi: 10.1155/2016/7462014. Epub 2016 Jan 10. Int J Biomed Imaging. 2016. PMID: 26880871 Free PMC article. Review. - Phosphorylation of amyloid beta (Aβ) peptides - a trigger for formation of toxic aggregates in Alzheimer's disease.
Kumar S, Walter J. Kumar S, et al. Aging (Albany NY). 2011 Aug;3(8):803-12. doi: 10.18632/aging.100362. Aging (Albany NY). 2011. PMID: 21869458 Free PMC article. - Caenorhabditis elegans hub genes that respond to amyloid beta are homologs of genes involved in human Alzheimer's disease.
Godini R, Pocock R, Fallahi H. Godini R, et al. PLoS One. 2019 Jul 10;14(7):e0219486. doi: 10.1371/journal.pone.0219486. eCollection 2019. PLoS One. 2019. PMID: 31291334 Free PMC article.
References
- Akiyama H. Abeta, tau and alpha-synuclein and glial cells. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006;26:23–31. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Armstrong RA, Cairns NJ, Myers D, Smith CU, Lantos PL, Rossor MN. A comparison of beta-amyloid deposition in the medial temporal lobe in sporadic Alzheimer's disease, Down's syndrome and normal elderly brains. Neurodegeneration. 1996;5:35–41. - PubMed
- Assefa Z, Valius M, Vantus T, Agostinis P, Merlevede W, Vandenheede JR. JNK/SAPK activation by platelet-derived growth factor in A431 cells requires both the phospholipase C-gamma and the phosphatidylinositol 3-kinase signaling pathways of the receptor. Biochem Biophys Res Commun. 1999;261:641–645. - PubMed
- Atwood CS, Perry G, Smith MA. Cerebral hemorrhage and amyloid-beta. Science. 2003;299:1014. author reply 1014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources