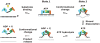Structure of the Yeast DEAD box protein Mss116p reveals two wedges that crimp RNA - PubMed (original) (raw)
Structure of the Yeast DEAD box protein Mss116p reveals two wedges that crimp RNA
Mark Del Campo et al. Mol Cell. 2009.
Abstract
The yeast DEAD box protein Mss116p is a general RNA chaperone that functions in mitochondrial group I and II intron splicing, translational activation, and RNA end processing. Here we determined high-resolution X-ray crystal structures of Mss116p complexed with an RNA oligonucleotide and ATP analogs AMP-PNP, ADP-BeF(3)(-), or ADP-AlF(4)(-). The structures show the entire helicase core acting together with a functionally important C-terminal extension. In all structures, the helicase core is in a closed conformation with a wedge alpha helix bending RNA 3' of the central bound nucleotides, as in previous DEAD box protein structures. Notably, Mss116p's C-terminal extension also bends RNA 5' of the central nucleotides, resulting in RNA crimping. Despite reported functional differences, we observe few structural changes in ternary complexes with different ATP analogs. The structures constrain models of DEAD box protein function and reveal a strand separation mechanism in which a protein uses two wedges to act as a molecular crimper.
Figures
Figure 1
Schematics showing domains in the DEAD-box proteins S_. cerevisiae_ Mss116p, N. crassa CYT-19, D. melanogaster Vasa, human DDX19, and human eIF4AIII. All of the proteins contain closely related helicase cores comprised of two RecA-like domains (domains 1 and 2) joined by a flexible linker. Mt, mitochondrial targeting sequence; NTE, N-terminal extension; CTE, C-terminal extension; Tail, hydrophilic tail based on hydropathy plots; the hydrophilic tail is basic in Mss116p and CYT-19 and acidic in Vasa (Mohr et al., 2008). The colored regions show portions of the NTE (yellow), domain 1 (blue), domain 2 (green), and CTE (orange) that have been visualized in the crystal structures of Mss116p (PDB ID 3i5x), Vasa (2db3), DDX19 (3fht), and eIF4AIII (2hyi). The domains are not drawn to scale.
Figure 2
Structure of Mss116p/Δ598-664 complexed with ssRNA and AMP-PNP. (A) Cartoon of Mss116p/Δ598-664 showing two views of the structure with domains colored as in Figure 1. The U10 RNA (yellow) and AMP-PNP (red) are shown in stick representation. (B) Location of DEAD-box protein motifs in the Mss116p structure. The views are the same as in (A), the motifs are colored (Q, purple; I, teal; Ia, light blue; GG, red; Ib, brown; II, orange; III, black; IV, green; QxxR, pink; V, peach; VI, yellow), and the RNA and AMP-PNP are dark gray.
Figure 3
Structure of the ATP-binding site of Mss116p/Δ598-664 with different bound ATP analogs. (A–C) Stereoviews of the ATP-binding site showing sigmaA-weighted m|Fo|− D|Fc| electron density contoured at 5 σ (gray) for an octahedrally coordinated Mg2+ ion, the putative catalytic (Wc) and relay (Wr) water molecules, and (A) AMP-PNP, (B) ADP-BeF3−, or (C) ADP-AlF4−. The ATP analog, Mg2+, and waters were removed from each model prior to map calculation. Mss116p is shown as a cartoon with side chains of residues that contact the ATP analog shown as sticks. (D–F) Details of coordination for (D) AMP-PNP, (E) ADP-BeF3−, and (F) ADP-AlF4−. In all panels, motif residues are colored as in Figure 2B, and ATP analog atoms are colored as follows: C (yellow), N (blue), O (red), P (orange), Be (purple), Al (gray), F (light blue), Mg (green). The Mg2+ ion and waters are shown as spheres.
Figure 4
RNA binding and bending by Mss116p. (A–D) Stereoviews of the RNA-binding cleft. (A) Molecular surface of Mss116p colored by electrostatic potential (blue is positive and red is negative) with the APBS plugin (Baker et al., 2001) in PyMOL. (B) Residues on the surface of Mss116p that contact U10 RNA. Residues are colored according to the conserved DEAD-box protein motif to which they belong or dark gray if not part of a conserved motif (see panel E). A portion of a symmetry related molecule (containing R584 and α20) that contacts the RNA is shown in transparent magenta. (C) Comparison of poly(U) RNAs bound to Mss116p (yellow), Vasa (red), eIF4AIII (white), and DDX19 (black). The RNA is shown after superposing the protein models of Vasa, eIF4AIII, and DDX19 onto Mss116p using PyMOL. The surface of Mss116p is colored by domains (see Figure 1) and is transparent to show the two α-helices (α8 and α18) involved in RNA bending. (D) Comparison of U10 RNA (yellow) bound to Mss116p (colored by domains) with an idealized A-form RNA duplex composed of a poly(U)10 strand (red) and a poly(A)10 strand (black). Regions that are involved in RNA bending (motif Ib and the CTE) or clash with the modeled poly(A) strand (Post-II) are labeled. (E) Cartoon showing contacts between Mss116p residues and U10 RNA. H-bonds, ionic contacts, hydrophobic contacts, and contacts through a water molecule are indicated by blue, light gray, black, and red arrows, respectively. Side- and main-chain interactions are indicated by solid and dashed lines, respectively. Double rectangles between RNA bases indicate base stacking. Residue colors are as in panel (B).
Figure 5
Model for the mechanism of RNA-strand separation by DEAD-box proteins. The DEAD-box protein with the helicase core in an open conformation binds ATP (yellow) and duplex RNA, leading to a partially closed pre-unwound state (state 1). State 1 then undergoes a conformational change to the closed unwound state (state 2) in which one RNA strand is bent or crimped. This conformational change is driven at least in part by the binding of one RNA strand in the RNA-binding cleft created by the interface of helicase core domains 1 and 2, and it actively unwinds the RNA duplex. ATP hydrolysis can occur during or after the conformational change from state 1 to state 2, with an additional conformational change required for the release of ADP + Pi (red) and the bound RNA strand. If the strands are incompletely separated or reassociate to reform the duplex, additional cycles of RNA binding and ATP hydrolysis may be needed for strand separation.
Similar articles
- Function of the C-terminal domain of the DEAD-box protein Mss116p analyzed in vivo and in vitro.
Mohr G, Del Campo M, Mohr S, Yang Q, Jia H, Jankowsky E, Lambowitz AM. Mohr G, et al. J Mol Biol. 2008 Feb 1;375(5):1344-64. doi: 10.1016/j.jmb.2007.11.041. Epub 2007 Nov 22. J Mol Biol. 2008. PMID: 18096186 Free PMC article. - Crystallization and preliminary X-ray diffraction of the DEAD-box protein Mss116p complexed with an RNA oligonucleotide and AMP-PNP.
Del Campo M, Lambowitz AM. Del Campo M, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009 Aug 1;65(Pt 8):832-5. doi: 10.1107/S1744309109027225. Epub 2009 Jul 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009. PMID: 19652352 Free PMC article. - High-throughput genetic identification of functionally important regions of the yeast DEAD-box protein Mss116p.
Mohr G, Del Campo M, Turner KG, Gilman B, Wolf RZ, Lambowitz AM. Mohr G, et al. J Mol Biol. 2011 Nov 11;413(5):952-72. doi: 10.1016/j.jmb.2011.09.015. Epub 2011 Sep 16. J Mol Biol. 2011. PMID: 21945532 Free PMC article. - Mss116p: a DEAD-box protein facilitates RNA folding.
Sachsenmaier N, Waldsich C. Sachsenmaier N, et al. RNA Biol. 2013 Jan;10(1):71-82. doi: 10.4161/rna.22492. Epub 2012 Oct 12. RNA Biol. 2013. PMID: 23064153 Free PMC article. Review. - Fluorescence methods in the investigation of the DEAD-box helicase mechanism.
Andreou AZ, Klostermeier D. Andreou AZ, et al. Exp Suppl. 2014;105:161-92. doi: 10.1007/978-3-0348-0856-9_8. Exp Suppl. 2014. PMID: 25095995 Review.
Cited by
- Cofactor-dependent specificity of a DEAD-box protein.
Young CL, Khoshnevis S, Karbstein K. Young CL, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2668-76. doi: 10.1073/pnas.1302577110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630256 Free PMC article. - The histone H4 tail regulates the conformation of the ATP-binding pocket in the SNF2h chromatin remodeling enzyme.
Racki LR, Naber N, Pate E, Leonard JD, Cooke R, Narlikar GJ. Racki LR, et al. J Mol Biol. 2014 May 15;426(10):2034-44. doi: 10.1016/j.jmb.2014.02.021. Epub 2014 Mar 4. J Mol Biol. 2014. PMID: 24607692 Free PMC article. - Yeast DEAD box protein Mss116p is a transcription elongation factor that modulates the activity of mitochondrial RNA polymerase.
Markov DA, Wojtas ID, Tessitore K, Henderson S, McAllister WT. Markov DA, et al. Mol Cell Biol. 2014 Jul;34(13):2360-9. doi: 10.1128/MCB.00160-14. Epub 2014 Apr 14. Mol Cell Biol. 2014. PMID: 24732805 Free PMC article. - RNA helicase proteins as chaperones and remodelers.
Jarmoskaite I, Russell R. Jarmoskaite I, et al. Annu Rev Biochem. 2014;83:697-725. doi: 10.1146/annurev-biochem-060713-035546. Epub 2014 Mar 12. Annu Rev Biochem. 2014. PMID: 24635478 Free PMC article. Review. - Crystal structure, mutational analysis and RNA-dependent ATPase activity of the yeast DEAD-box pre-mRNA splicing factor Prp28.
Jacewicz A, Schwer B, Smith P, Shuman S. Jacewicz A, et al. Nucleic Acids Res. 2014 Nov 10;42(20):12885-98. doi: 10.1093/nar/gku930. Epub 2014 Oct 10. Nucleic Acids Res. 2014. PMID: 25303995 Free PMC article.
References
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, Pedersen JS, Séraphin B, Le Hir H, Andersen GR. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–1972. - PubMed
- Bono F, Ebert J, Lorentzen E, Conti E. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell. 2006;126:713–725. - PubMed
- CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32-GM76961/GM/NIGMS NIH HHS/United States
- R37 GM037951/GM/NIGMS NIH HHS/United States
- R01 GM037951/GM/NIGMS NIH HHS/United States
- F32 GM076961/GM/NIGMS NIH HHS/United States
- GM037951/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous