Identifying genetic determinants needed to establish a human gut symbiont in its habitat - PubMed (original) (raw)
Identifying genetic determinants needed to establish a human gut symbiont in its habitat
Andrew L Goodman et al. Cell Host Microbe. 2009.
Abstract
The human gut microbiota is a metabolic organ whose cellular composition is determined by a dynamic process of selection and competition. To identify microbial genes required for establishment of human symbionts in the gut, we developed an approach (insertion sequencing, or INSeq) based on a mutagenic transposon that allows capture of adjacent chromosomal DNA to define its genomic location. We used massively parallel sequencing to monitor the relative abundance of tens of thousands of transposon mutants of a saccharolytic human gut bacterium, Bacteroides thetaiotaomicron, as they established themselves in wild-type and immunodeficient gnotobiotic mice, in the presence or absence of other human gut commensals. In vivo selection transforms this population, revealing functions necessary for survival in the gut: we show how this selection is influenced by community composition and competition for nutrients (vitamin B(12)). INSeq provides a broadly applicable platform to explore microbial adaptation to the gut and other ecosystems.
Figures
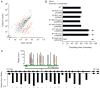
Figure 1. Mapping and quantifying tens of thousands of transposon insertion strains by high-throughput insertion sequencing (INSeq)
(A) A negative selection scheme for identification of genes required for colonization in vivo. Mutants in genes important for competitive growth (red) are expected to decrease in relative abundance in the output population. (B) Preparation of an INSeq library. Genomic DNA is extracted from the mutagenized bacterial population, digested with MmeI, and separated by polyacrylamide gel electrophoresis (PAGE). Transposon-sized fragments are appended with double-stranded oligonucleotide adapters by ligation. Limited cycles of PCR create the final library molecules for sequencing. (C) Map of insertion sites in the B. thetaiotaomicron genome. An arrow marks the origin of replication. (D) Reproducibility of library preparation and sequencing protocols. Technical replicates were prepared and sequenced from a single transposon mutant population. Each point represents the abundance of insertions in a single gene; the coefficient of determination, R2, on log-transformed abundance values is 0.92.
Figure 2. Mapping an archived strain collection by combinatorial pooling and high-throughput sequencing
(A) Individual strains are archived in a 96-well format and then placed into a subset of 24 pools according to a unique 24-bit binary string assigned to each strain. Sub-libraries are prepared from each of these pools using one of 24 pool-specific barcoded dsDNA adapters in the ligation step. Sub-libraries are then combined and sequenced in a single run. Reads mapping to a specific insertion location are compiled and the associated pool-specific barcodes are identified to recreate the 24-bit string (and with it, the physical location of the corresponding strain in the archived collection). (B) Sample pool distribution patterns for archived strains. Strains are placed in pools designated with white boxes and omitted from pools marked with black boxes. The 13,000 patterns generated are each distinct in at least six positions and do not overlap to produce another pattern in the set. (C) Confirmation of strain-insertion assignments.
Figure 3. Identification of genetic determinants of fitness in vivo
(A) The transposon mutant population is largely stable in vivo. The relative abundance of mutations in each gene (points) was compared between input and output (median from wild-type monoassociated mice, n = 15) populations. Genes that show a statistically significant change (q<0.05) in representation in all three cohorts of mice are shown in red, others in gray. The relative abundances of 80 gene-sized ‘neutral loci’ are shown in black (no significant change) and green (three ‘neutral loci’ that pass the significance criteria). (B) Individual strains retrieved from the archived collection demonstrate that genes critical for fitness in vivo are dispensable in vitro. Each strain was cultured individually in TYG medium and doubling time was calculated from OD600 measurements. Mutants predicted by INSeq to have in vitro growth defects are marked with arrows. N/D, no growth detected. rnf, Na+-transporting NADH:ubiquinone oxidoreductase (BT0616-22); S-layer, putative S-layer locus (BT1953-7); nqr, Na+-translocating NADH-quinone reductase (BT1155-60); hly, hemolysin A (BT3459). (C) Insertions in the CPS4 locus (BT1338-58) show a consistent in vivo fitness defect. Individual insertion locations (open arrowheads) in a representative gene (BT1346; green arrow) are shown at top. Read counts for input (black) and output (orange) samples at each insertion location are indicated (output counts represent the median from the ceca of wild-type monoassociated mice, n = 15). Median output:input ratios for each gene (black/green arrows) across the CPS4 locus are shown below. Asterisks indicate the average FDR-corrected _p_-value (q) across 3 experimental cohorts (n = 5 mice/cohort): *q< 0.05; **q< 0.01.
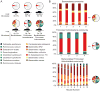
Figure 4. Identification of environmental and microbial factors that determine the fitness landscape for B. thetaiotaomicron
(A) Shifts in the population structure of the ~35,000-strain B. thetaiotaomicron mutant population reflect alterations in the selective pressures on its genome. The relative abundance of each mutant in the B. thetaiotaomicron population was evaluated in multiple host genotypes (wild-type C57Bl/6; _Rag1_−/−; _Myd88_−/−) and microbial contexts. (B) qPCR assays of cecal microbial community composition in gnotobiotic mice at the time of sacrifice. The ~35,000-strain B. thetaiotamicron population is indicated with arrows.
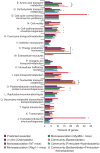
Figure 5. COG category-based classification of genes critical for fitness in vitro and in vivo
Percent representation was calculated as (number of genes in a COG category/number of genes underrepresented in output population). Significant enrichments in specific COG categories, assessed by comparing these percentages to a null expectation based on the size of the gene list and the representation of a given category in the genome, are marked with asterisks (Benjamini-Hochberg corrected p<0.05).
Figure 6. Clustering of B. thetaiotaomicron mutant population structures after manipulation of host environment or microbial context
(A) Unsupervised hierarchical clustering of B. thetaiotaomicron mutant population structures in vitro and in vivo. Branches are colored by treatment. Pie charts indicate the microbial context of the mutant population in samples from different branches of the tree. Bootstrap support is indicated by a square at each node: >50% (white), >90% (yellow), >99% (black/unmarked). (B) Principal coordinates analysis based on the representation of transposon-disrupted genes.
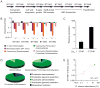
Figure 7. Community context modulates the fitness requirement for a vitamin B12-regulated locus in B. thetaiotaomicron
(A) Genetic organization and predicted annotation of the BT1957-49 locus. (B) The relative abundance of transposon insertion mutants in input and output mutant populations is dependent on community context. Asterisks indicate FDR-corrected _p_-value (q) for each cohort: *q< 0.05; **q< 0.01. Few insertions/reads were identified for BT1952-49; fitness defects were similar in wildtype, _Rag1_−/−, and _Myd88_−/− monoassociated animals (data not shown). (C) BT1956 gene expression is regulated by vitamin B12. Error bars represent one standard deviation based on triplicate qRT-PCR experiments. Similar results were observed for BT1954 (data not shown). Interestingly, BT1956 insertion mutants do not exhibit growth defects in medium containing either 37 nM or 0.37 nM B12, suggesting that multiple loci are involved in acquisition of this vitamin in vitro (data not shown). Consistent with this observation, other loci annotated as being potentially involved in B12 uptake were coordinately upregulated with BT1957-49 in the genome-wide transcriptional profiling experiments discussed above. (D) Pie charts showing that the defined microbial communities characterized in this study vary in their capacity for vitamin B12 biosynthesis. Color codes: dark blue, species with a predicted complete biosynthetic pathway (see Tables S16 and S17 for annotations) that are able to grow in defined medium lacking B12 (‘demonstrated prototrophs’); light blue, organisms with a predicted complete biosynthetic pathway able to grow on rich medium but not on defined medium with or without B12 (‘predicted prototrophs’); dark green, species without a complete pathway whose growth in defined medium requires B12 (‘demonstrated auxotrophs’); light green, species without a complete biosynthetic pathway unable to grow on defined medium with or without B12 (‘predicted auxotrophs’); black, species that do not grow on defined medium with or without B12 but that possess a B12-independent methionine synthase (the presence of such an enzyme implies the absence of a B12 requirement). The relative proportions of prototrophs and auxotrophs shown represent the average in each community in vivo as determined by species-specific qPCR of cecal contents. (E) The B. thetaiotaomicron fitness requirement for BT1957-3 correlates with levels of the B12-prototrophic species Ruminococcus obeum in the microbial community. Each point represents an individual mouse containing a defined multi-species microbiota in addition to the B. thetaiotaomicron transposon mutant population. Color code: blue, Firmicutes+Actinobacteria community; pink, Bacteroidetes+Firmicutes+Actinobacteria. The relative abundance of R. obeum (determined by qPCR analysis of cecal contents at the time of sacrifice) is plotted against the average output:input ratio of B. thetaiotaomicron transposon mutants in genes BT1957-3 in each individual.
Similar articles
- Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron.
Benjdia A, Martens EC, Gordon JI, Berteau O. Benjdia A, et al. J Biol Chem. 2011 Jul 22;286(29):25973-82. doi: 10.1074/jbc.M111.228841. Epub 2011 Apr 20. J Biol Chem. 2011. PMID: 21507958 Free PMC article. - Bacterial colonization factors control specificity and stability of the gut microbiota.
Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Lee SM, et al. Nature. 2013 Sep 19;501(7467):426-9. doi: 10.1038/nature12447. Epub 2013 Aug 18. Nature. 2013. PMID: 23955152 Free PMC article. - Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome.
McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, Gordon JI. McNulty NP, et al. PLoS Biol. 2013;11(8):e1001637. doi: 10.1371/journal.pbio.1001637. Epub 2013 Aug 20. PLoS Biol. 2013. PMID: 23976882 Free PMC article. - A genomic view of our symbiosis with members of the gut microbiota.
Gordon JI. Gordon JI. J Pediatr Gastroenterol Nutr. 2005 Apr;40 Suppl 1:S28. doi: 10.1097/00005176-200504001-00016. J Pediatr Gastroenterol Nutr. 2005. PMID: 15805839 Review. No abstract available. - An insider's perspective: Bacteroides as a window into the microbiome.
Wexler AG, Goodman AL. Wexler AG, et al. Nat Microbiol. 2017 Apr 25;2:17026. doi: 10.1038/nmicrobiol.2017.26. Nat Microbiol. 2017. PMID: 28440278 Free PMC article. Review.
Cited by
- The enigmatic biology of rickettsiae: recent advances, open questions and outlook.
McGinn J, Lamason RL. McGinn J, et al. Pathog Dis. 2021 Apr 9;79(4):ftab019. doi: 10.1093/femspd/ftab019. Pathog Dis. 2021. PMID: 33784388 Free PMC article. Review. - Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation.
Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. Cullen TW, et al. Science. 2015 Jan 9;347(6218):170-5. doi: 10.1126/science.1260580. Science. 2015. PMID: 25574022 Free PMC article. - Cofitness network connectivity determines a fuzzy essential zone in open bacterial pangenome.
Zhang P, Zhang B, Ji YY, Jiao J, Zhang Z, Tian CF. Zhang P, et al. mLife. 2024 Jun 28;3(2):277-290. doi: 10.1002/mlf2.12132. eCollection 2024 Jun. mLife. 2024. PMID: 38948139 Free PMC article. - Pangenome evaluation of gene essentiality in Streptococcus pyogenes.
Jespersen MG, Hayes AJ, Tong SYC, Davies MR. Jespersen MG, et al. Microbiol Spectr. 2024 Aug 6;12(8):e0324023. doi: 10.1128/spectrum.03240-23. Epub 2024 Jul 16. Microbiol Spectr. 2024. PMID: 39012116 Free PMC article. - Genome-wide quantification of contributions to sexual fitness identifies genes required for spore viability and health in fission yeast.
Billmyre RB, Eickbush MT, Craig CJ, Lange JJ, Wood C, Helston RM, Zanders SE. Billmyre RB, et al. PLoS Genet. 2022 Oct 27;18(10):e1010462. doi: 10.1371/journal.pgen.1010462. eCollection 2022 Oct. PLoS Genet. 2022. PMID: 36301993 Free PMC article.
References
- Breiman L. Random Forests. Machine Learning. 2001;45:5–32.
Publication types
MeSH terms
Grants and funding
- 1F32AI078628-01/AI/NIAID NIH HHS/United States
- R01 DK030292/DK/NIDDK NIH HHS/United States
- DK30292/DK/NIDDK NIH HHS/United States
- F32 AI078628-01/AI/NIAID NIH HHS/United States
- R37 DK030292/DK/NIDDK NIH HHS/United States
- R37 DK030292-29/DK/NIDDK NIH HHS/United States
- F32 AI078628/AI/NIAID NIH HHS/United States
- F32 AI078628-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials