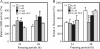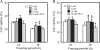Insufficiency of copper ion homeostasis causes freeze-thaw injury of yeast cells as revealed by indirect gene expression analysis - PubMed (original) (raw)
Insufficiency of copper ion homeostasis causes freeze-thaw injury of yeast cells as revealed by indirect gene expression analysis
Shunsuke Takahashi et al. Appl Environ Microbiol. 2009 Nov.
Abstract
Saccharomyces cerevisiae is exposed to freeze-thaw stress in commercial processes, including frozen dough baking. Cell viability and fermentation activity after a freeze-thaw cycle were dramatically decreased due to freeze-thaw injury. Because this type of injury involves complex phenomena, the injury mechanisms are not fully understood. We examined freeze-thaw injury by indirect gene expression analysis during postthaw incubation after freeze-thaw treatment using DNA microarray profiling. The results showed that genes involved in the homeostasis of metal ions were frequently contained in genes that were upregulated, depending on the freezing period. We assessed the phenotype of deletion mutants of the metal ion homeostasis genes that exhibited freezing period-dependent upregulation and found that the strains with deletion of the MAC1 and CTR1 genes involved in copper ion homeostasis exhibited freeze-thaw sensitivity, suggesting that copper ion homeostasis is required for freeze-thaw tolerance. We found that supplementation with copper ions during postthaw incubation increased intracellular superoxide dismutase activity and intracellular levels of reactive oxygen species were decreased. Moreover, cell viability was increased by supplementation with copper ions. These results suggest that insufficiency of copper ion homeostasis may be one of the causes of freeze-thaw injury.
Figures
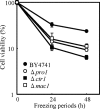
FIG. 1.
Changes in viable cell numbers (A) and intracellular levels of ROS (B) of strain BY4741 after freeze-thaw treatment. In panel A, viable cell numbers were measured after freeze-thaw treatment for 24 to 96 h. In panel B, the intracellular levels of ROS were measured after postthaw incubation preceded by freeze-thaw treatment for 24 to 96 h. The fluorescence intensity of the cells before freeze-thaw treatment was relatively set as 100%. The values are the means and standard deviations of results from three independent experiments.
FIG. 2.
The viabilities of strain BY4741 (as a positive control) and the Δ_mac1_, Δ_ctr1_, and Δ_pro1_ (as a negative control) deletion strains were measured after freeze-thaw treatment for 24 or 48 h. The values are means and standard deviations of results from three independent experiments.
FIG. 3.
Changes in intracellular SOD activity (A) and intracellular levels of ROS (B) in BY4741 by supplementation with copper ions during postthaw incubation. In panel A, the intracellular SOD activities were measured after postthaw incubation preceded by freeze-thaw treatment for 24 or 48 h using SD medium containing various copper ion concentrations. The unit of SOD activity of the cells before freeze-thaw treatment was relatively set as 100%. In panel B, the intracellular levels of ROS were measured after postthaw incubation preceded by freeze-thaw treatment for 24 or 48 h using SD medium containing various copper ion concentrations. The fluorescence intensity of the cells before freeze-thaw treatment was relatively set as 100%. The values are the means and standard deviations of results from three independent experiments. Asterisks and double asterisks indicate that there are significant differences between the values from samples supplemented with no copper and the values from respective samples with P values of <0.05 and <0.01, respectively.
FIG. 4.
Effects of supplementation with copper ions on cell viability of BY4741 after freeze-thaw treatment. The cell viabilities after freeze-thaw treatment were measured using YPD (A) and SD (B) agar media containing various copper ion concentrations. The viability of cells before freeze-thaw treatment was relatively set as 100%. The data shown are means of more than triplicate measurements from a representative experiment. Error bars represent the standard deviations of the means. Asterisks and double asterisks indicate that there are significant differences between the viabilities of samples supplemented with no copper and the viabilities of respective samples with P values of <0.05 and <0.01, respectively.
Similar articles
- Importance of Proteasome Gene Expression during Model Dough Fermentation after Preservation of Baker's Yeast Cells by Freezing.
Watanabe D, Sekiguchi H, Sugimoto Y, Nagasawa A, Kida N, Takagi H. Watanabe D, et al. Appl Environ Microbiol. 2018 May 31;84(12):e00406-18. doi: 10.1128/AEM.00406-18. Print 2018 Jun 15. Appl Environ Microbiol. 2018. PMID: 29625985 Free PMC article. - Identification and classification of genes required for tolerance to freeze-thaw stress revealed by genome-wide screening of Saccharomyces cerevisiae deletion strains.
Ando A, Nakamura T, Murata Y, Takagi H, Shima J. Ando A, et al. FEMS Yeast Res. 2007 Mar;7(2):244-53. doi: 10.1111/j.1567-1364.2006.00162.x. Epub 2006 Sep 21. FEMS Yeast Res. 2007. PMID: 16989656 - Cryopreservation and the Freeze-Thaw Stress Response in Yeast.
Cabrera E, Welch LC, Robinson MR, Sturgeon CM, Crow MM, Segarra VA. Cabrera E, et al. Genes (Basel). 2020 Jul 22;11(8):835. doi: 10.3390/genes11080835. Genes (Basel). 2020. PMID: 32707778 Free PMC article. Review. - Metal-ion regulation of gene expression in yeast.
Winge DR, Jensen LT, Srinivasan C. Winge DR, et al. Curr Opin Chem Biol. 1998 Apr;2(2):216-21. doi: 10.1016/s1367-5931(98)80063-x. Curr Opin Chem Biol. 1998. PMID: 9667925 Review.
Cited by
- The roles of reactive oxygen species and antioxidants in cryopreservation.
Len JS, Koh WSD, Tan SX. Len JS, et al. Biosci Rep. 2019 Aug 29;39(8):BSR20191601. doi: 10.1042/BSR20191601. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31371631 Free PMC article. Review. - Long-term study on survival and development of successive generations of Mytilus galloprovincialis cryopreserved larvae.
Heres P, Troncoso J, Paredes E. Heres P, et al. Sci Rep. 2022 Aug 10;12(1):13632. doi: 10.1038/s41598-022-17935-0. Sci Rep. 2022. PMID: 35948747 Free PMC article. - Local climatic adaptation in a widespread microorganism.
Leducq JB, Charron G, Samani P, Dubé AK, Sylvester K, James B, Almeida P, Sampaio JP, Hittinger CT, Bell G, Landry CR. Leducq JB, et al. Proc Biol Sci. 2014 Jan 8;281(1777):20132472. doi: 10.1098/rspb.2013.2472. Print 2014 Feb 22. Proc Biol Sci. 2014. PMID: 24403328 Free PMC article. - Role of Klotho as a Modulator of Oxidative Stress Associated with Ovarian Tissue Cryopreservation.
Kim B, Yoon H, Kim T, Lee S. Kim B, et al. Int J Mol Sci. 2021 Dec 17;22(24):13547. doi: 10.3390/ijms222413547. Int J Mol Sci. 2021. PMID: 34948343 Free PMC article.
References
- Aguilera, J., F. Randez-Gil, and J. A. Prieto. 2007. Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol. Rev. 31:327-341. - PubMed
- Ando, A., T. Nakamura, Y. Murata, H. Takagi, and J. Shima. 2007. Identification and classification of genes required for tolerance to freeze-thaw stress revealed by genome-wide screening of Saccharomyces cerevisiae deletion strains. FEMS Yeast Res. 7:244-253. - PubMed
- Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. - PubMed
- De Freitas, J. M., J. H. Kim, H. Poynton, T. Su, H. Wintz, T. Fox, P. Holman, A. Loguinov, S. Keles, M. van der Laan, and C. Vulpe. 2004. Exploratory and confirmatory gene expression profiling of mac1Δ. J. Biol. Chem. 279:4450-4458. - PubMed
- Du, X., and H. Takagi. 2005. N-Acetyltransferase Mpr1 confers freeze tolerance on Saccharomyces cerevisiae by reducing reactive oxygen species. J. Biochem. 138:391-397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials