Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR - PubMed (original) (raw)
Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR
Tetsuro Ikegami et al. Ann N Y Acad Sci. 2009 Sep.
Abstract
Rift Valley fever virus (RVFV), which belongs to the genus Phlebovirus, family Bunyaviridae, is a negative-stranded RNA virus carrying a single-stranded, tripartite RNA genome. RVFV is an important zoonotic pathogen transmitted by mosquitoes and causes large outbreaks among ruminants and humans in Africa and the Arabian Peninsula. Human patients develop an acute febrile illness, followed by a fatal hemorrhagic fever, encephalitis, or ocular diseases. A viral nonstructural protein, NSs, is a major viral virulence factor. Past studies showed that NSs suppresses the transcription of host mRNAs, including interferon-beta mRNAs. Here we demonstrated that the NSs protein induced post-transcriptional downregulation of dsRNA-dependent protein kinase (PKR), to prevent phosphorylation of eIF2alpha and promoted viral translation in infected cells. These two biological activities of the NSs most probably have a synergistic effect in suppressing host innate immune functions and facilitate efficient viral replication in infected mammalian hosts.
Figures
Figure 1
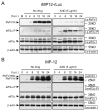
Effects of ActD on the replication of MP-12 and MP-12 lacking the NSs gene. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of the S segments of MP-12 and rMP12-rLuc. (B) Type I IFN-deficient VeroE6 cells were mock-treated or independently infected with MP-12 and rMP12-rLuc at an moi of 3, immediately treated with ActD (5 μg/ml) or left untreated, and culture fluids were harvested at 16 h.p.i. Virus titers of MP-12 and rMP12-rLuc were measured by a plaque assay. The virus replication of rMP12-rLuc was significantly reduced in the presence of ActD (*p<0.001; Student's _t_-test). Data are expressed as mean +/- standard deviation of three independent experiments.
Figure 2
Status of eIF2α phosphorylation in rMP12-rLuc-infected cells and MP-12-infected cells in the presence of transcriptional inhibitors. The Fig is adapted from Ikegami et al.[26] VeroE6 cells were mock infected (M) or infected with rMP12-rLuc (A) or MP-12 (B) at an moi of 3, and then immediately treated with ActD or left untreated (No drug). Samples were harvested at the indicated time points post infection for Western blot analysis. RVFV N protein, NSs proteins, phosphorylated eIF2α, total eIF2α, and β-actin are shown by arrowheads. The data are representative of three independent experiments.
Figure 3
Role of PKR in eIF2α phosphorylation in infected cells under transcriptional suppression. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of S segments of MP-12 and rMP12-PKRΔE7. (B) VeroE6 cells were independently infected with MP-12, rMP12-rLuc and rMP12-PKRΔE7 at an moi of 3, or were mock infected. Cells were immediately treated with ActD (Act) or 50 μg/ml of α-amanitin (Ama), or were untreated. Cell extracts were prepared at 16 h.p.i. for Western blot analysis. Western blot analysis showing the accumulation of eIF2α, phosphorylated eIF2α, N protein, NSs protein, Flag-PKRΔE7 and β-actin in infected VeroE6 cells.
Figure 4
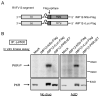
Autophosphorylation of PKR in infected cells. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of RVFV S segments of rMP12-NSs-Flag and rMP12-rLuc-Flag. (B) 293 cells were mock-infected or infected with rMP12-NSs-Flag, rMP12-rLuc-Flag or rMP12-PKRΔE7 at an moi of 3, and, then, cells were mock-treated (No drug) or immediately treated with ActD. A cytoplasmic fraction was collected at 16 h.p.i. and the IP-kinase assay of PKR was performed as described previously.[26] A portion of the samples were used for Western blot analysis by using anti-PKR monoclonal antibody to show the abundance of immunoprecipitated PKR (bottom panel).
Figure 5
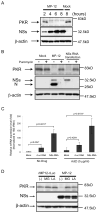
Analysis of NSs-induced PKR downregulation. The Fig is adapted from Ikegami et al.[26] (A) 293 cells were mock infected (Mock) or infected with MP-12 (MP-12) at an moi of 3. Whole-cell lysates were collected at 2, 4, 6 and 8 h.p.i. Anti-PKR antibody, anti-NSs antibody and anti-β-actin antibody were used to detect PKR, NSs and β-actin, respectively. (B) 293 cells were mock infected (Mock) or infected with MP-12 at an moi of 3 or transfected with in vitro-synthesized RNA transcripts encoding NSs. Cells were then mock-treated or treated with 100μg/ml of puromycin. Cell extracts were harvested at 16 h.p.i. or 16 h post transfection, and the abundance of PKR and viral proteins were analyzed by Western blotting with anti-PKR antibody (top panel), anti-RVFV antibody (middle panel) or anti-β-actin antibody (bottom panel). (C) 293 cells were mock-transfected or transfected with in vitro-synthesized RNA transcripts encoding MP-12 NSs or rLuc. Cells were mock-treated or treated with 5 μg/ml of ActD. Total RNA was harvested at 8 h post transfection, and analyzed by real-time PCR. The relative abundance of PKR mRNA of each sample was calculated by the ΔΔCT method based on the abundance of 18S ribosomal RNA. The data shown in the graph (mean +/- standard deviation) were obtained from three independent experiments. The p value was determined by Student's _t_-test (*: p<0.05). (D) 293 cells were infected with rMP12-rLuc or MP-12 at an moi of 3, and, then, treated with 10 μM of MG132 (MG) or 50 μM of lactacystin (LA) or they were mock treated (-). Whole-cell lysates were collected at 8 h.p.i. and the abundance of PKR, NSs and β-actin was examined by Western blot analysis.
Similar articles
- Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation.
Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, Makino S. Ikegami T, et al. PLoS Pathog. 2009 Feb;5(2):e1000287. doi: 10.1371/journal.ppat.1000287. Epub 2009 Feb 6. PLoS Pathog. 2009. PMID: 19197350 Free PMC article. - NSs Virulence Factor of Rift Valley Fever Virus Engages the F-Box Proteins FBXW11 and β-TRCP1 To Degrade the Antiviral Protein Kinase PKR.
Kainulainen M, Lau S, Samuel CE, Hornung V, Weber F. Kainulainen M, et al. J Virol. 2016 Jun 10;90(13):6140-7. doi: 10.1128/JVI.00016-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27122577 Free PMC article. - Mechanistic Insight into the Host Transcription Inhibition Function of Rift Valley Fever Virus NSs and Its Importance in Virulence.
Terasaki K, Ramirez SI, Makino S. Terasaki K, et al. PLoS Negl Trop Dis. 2016 Oct 6;10(10):e0005047. doi: 10.1371/journal.pntd.0005047. eCollection 2016 Oct. PLoS Negl Trop Dis. 2016. PMID: 27711108 Free PMC article. - [Rift Valley fever virus].
Ikegami T, Makino S. Ikegami T, et al. Uirusu. 2004 Dec;54(2):229-35. doi: 10.2222/jsv.54.229. Uirusu. 2004. PMID: 15745161 Review. Japanese. - The pathogenesis of Rift Valley fever.
Ikegami T, Makino S. Ikegami T, et al. Viruses. 2011 May;3(5):493-519. doi: 10.3390/v3050493. Viruses. 2011. PMID: 21666766 Free PMC article. Review.
Cited by
- Use of Human Macrophages to Study Bunyavirus NSs Functions.
Alkan C, Ikegami T. Alkan C, et al. Methods Mol Biol. 2024;2824:397-408. doi: 10.1007/978-1-0716-3926-9_25. Methods Mol Biol. 2024. PMID: 39039426 - Use of Single-Domain Antibodies Against the NSm Protein for the Detection of Cells Infected by Rift Valley Fever Virus.
Romanet C, Tamietti C, Mériaux V, Bontems F, Montagutelli X, Lafaye P, Flamand M. Romanet C, et al. Methods Mol Biol. 2024;2824:147-164. doi: 10.1007/978-1-0716-3926-9_11. Methods Mol Biol. 2024. PMID: 39039412 - Rift Valley Fever Virus Encephalitis: Viral and Host Determinants of Pathogenesis.
Wilson LR, McElroy AK. Wilson LR, et al. Annu Rev Virol. 2024 Sep;11(1):309-325. doi: 10.1146/annurev-virology-093022-011544. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38635867 Review. - Rift Valley Fever Virus Nucleoprotein Triggers Autophagy to Dampen Antiviral Innate Immune Responses.
Zhu X, Guan Z, Fang Y, Zhang Y, Guan Z, Li S, Peng K. Zhu X, et al. J Virol. 2023 Apr 27;97(4):e0181422. doi: 10.1128/jvi.01814-22. Epub 2023 Mar 20. J Virol. 2023. PMID: 36939341 Free PMC article. - Intact Type I Interferon Receptor Signaling Prevents Hepatocellular Necrosis but Not Encephalitis in a Dose-Dependent Manner in Rift Valley Fever Virus Infected Mice.
Michaely LM, Schuwerk L, Allnoch L, Schön K, Waltl I, Larsen PK, Pavlou A, Prajeeth CK, Rimmelzwaan GF, Becker SC, Kalinke U, Baumgärtner W, Gerhauser I. Michaely LM, et al. Int J Mol Sci. 2022 Oct 18;23(20):12492. doi: 10.3390/ijms232012492. Int J Mol Sci. 2022. PMID: 36293352 Free PMC article.
References
- Morvan J, Saluzzo JF, Fontenille D, et al. Rift Valley fever on the east coast of Madagascar. Res Virol. 1991;142:475–482. - PubMed
- Peters CJ. Handbook Series of Zoonoses, Section B: Viral Zoonoses. Vol. 1. Boca Raton, FL: CRC Press; 1981. pp. 403–420.
- Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian Peninsula. Int J Antimicrob Agents. 2003;21:153–157. - PubMed
- Meegan JM. The Rift Valley fever epizootic in Egypt 1977-78. 1. Description of the epizootic and virological studies. Trans R Soc Trop Med Hyg. 1979;73:618–623. - PubMed
- Al-Hazmi A, Al-Rajhi AA, Abboud EB, et al. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology. 2005;112:313–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous