Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini - PubMed (original) (raw)
Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini
Edwin C Thrower et al. Pancreas. 2009 Nov.
Abstract
Objectives: To define the role of protein kinase C delta (PKC delta) in acinar cell responses to the hormone cholecystokinin-8 (CCK) using isoform-specific inhibitors and a previously unreported genetic deletion model.
Methods: Pancreatic acinar cells were isolated from (1) rat, and pretreated with a PKC delta-specific inhibitor or (2) PKC delta-deficient and wild type mice. Isolated cells were stimulated with CCK (0.001-100 nmol/L) and cell responses were measured.
Results: The PKC delta inhibitor did not affect stimulated amylase secretion from rat pancreatic acinar cells. Cholecystokinin-8 stimulation induced a typical biphasic dose-response curve for amylase secretion in acinar cells isolated from both PKC delta(-/-) and wild type mice, with maximal stimulation at 10-pmol/L CCK. Cholecystokinin-8 (100 nmol/L) induced zymogen and nuclear factor kappaB activation in both PKC delta(-/-) and wild type mice, although it was up to 50% less in PKC delta(-/-).
Conclusions: In contrast to previous studies, this study has used specific and complementary approaches to examine PKC delta-mediated acinar cell responses. We could not confirm that it mediates amylase release but corroborated its role in the early stages of acute pancreatitis.
Figures
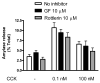
Figure 1. Rottlerin but not GF109203X affect CCK-induced amylase release
Isolated acinar cells were pre-incubated with or without broad-spectrum PKC inhibitors GF109203X and rottlerin (10 μM) for 2 hours, followed by 30 min CCK treatment (0.1nM and 100nM). Amylase release is expressed as % total release [medium/(medium + cells)]. N=3; *p<0.05
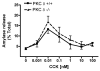
Figure 2. Isoform-specific PKC δ translocation inhibitor does not affect CCK-induced amylase release
Isolated acinar cells were pre-incubated with or without PKC δ translocation inhibitor (10 μM) for 2 hours, followed by a) 30 min CCK treatment over a concentration range (0.001nM - 100nM); b) 100 nM CCK treatment at set time points (0, 5, 15, 30 min). Amylase release is expressed as % total release [medium/(medium + cells)]. N=3.
Figure 2. Isoform-specific PKC δ translocation inhibitor does not affect CCK-induced amylase release
Isolated acinar cells were pre-incubated with or without PKC δ translocation inhibitor (10 μM) for 2 hours, followed by a) 30 min CCK treatment over a concentration range (0.001nM - 100nM); b) 100 nM CCK treatment at set time points (0, 5, 15, 30 min). Amylase release is expressed as % total release [medium/(medium + cells)]. N=3.
Figure 3. Genetic deletion of PKC δ does not affect expression levels of other PKC isoforms or production of digestive enzymes in mouse acinar cells
Pancreata from PKC δ -/- and PKC δ +/+ were removed and protein extracts processed for Western blot analysis. Blots were probed with antibodies for A) PKC α, δ, ε and ζ isoforms and B) amylase, trypsinogen and GAPDH; representative blots are shown. These results confirm the absence of PKC δ in the knockout animals and that genetic deletion of PKC δ did not affect the expression of other PKC isoforms or the production of digestive enzymes.
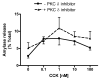
Figure 4. CCK- and carbachol-induced amylase release is not reduced in PKCδ -/- mice versus wild type (PKCδ +/+)
Pancreatic acinar cells were isolated from PKC δ -/- and +/+ mice and then stimulated for 30 min with a) CCK (0.001-100 nM); b) carbachol (0.01-100 μM). Amylase release is expressed as % total release [medium/(medium + cells)]. N=5.
Figure 4. CCK- and carbachol-induced amylase release is not reduced in PKCδ -/- mice versus wild type (PKCδ +/+)
Pancreatic acinar cells were isolated from PKC δ -/- and +/+ mice and then stimulated for 30 min with a) CCK (0.001-100 nM); b) carbachol (0.01-100 μM). Amylase release is expressed as % total release [medium/(medium + cells)]. N=5.
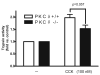
Figure 5. CCK-induced trypsinogen activation is reduced in PKCδ -/- mice versus wild type (PKCδ +/+)
Pancreatic acinar cells were isolated from PKC δ -/- and +/+ animals and then stimulated for 30 min with 100 nM CCK. Trypsin activity is normalized to amylase content and expressed as fold vs. control. N=6. A reduction in trypsin activity is seen in the knockout animals approaching significance.
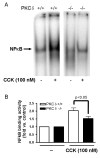
Figure 6. CCK-induced NFκB activation is reduced in PKCδ -/- mice versus wild type (PKCδ +/+)
Pancreatic acinar cells were isolated from PKC δ -/- and +/+ animals and then stimulated for 30 min with 100 nM CCK. A) NFκB binding activity was measured in nuclear extracts by EMSA. B) NFκB band intensities were quantified in the PhosphorImager and normalized on the band intensity in the corresponding unstimulated control acini. Values are means +/-SEM (N= 5).
Similar articles
- Regulation of CCK-induced amylase release by PKC-delta in rat pancreatic acinar cells.
Li C, Chen X, Williams JA. Li C, et al. Am J Physiol Gastrointest Liver Physiol. 2004 Oct;287(4):G764-71. doi: 10.1152/ajpgi.00111.2004. Epub 2004 Jun 24. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15217780 - Involvement of myristoylated alanine-rich C kinase substrate phosphorylation and translocation in cholecystokinin-induced amylase release in rat pancreatic acini.
Satoh K, Narita T, Katsumata-Kato O, Sugiya H, Seo Y. Satoh K, et al. Am J Physiol Gastrointest Liver Physiol. 2016 Mar 15;310(6):G399-409. doi: 10.1152/ajpgi.00198.2015. Epub 2016 Jan 7. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26744470 - Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism.
Tapia JA, Jensen RT, García-Marín LJ. Tapia JA, et al. Biochim Biophys Acta. 2006 Jan;1763(1):25-38. doi: 10.1016/j.bbamcr.2005.10.007. Epub 2005 Nov 18. Biochim Biophys Acta. 2006. PMID: 16364465 - PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells.
Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR Jr, Shimosegawa T, Pandol SJ. Satoh A, et al. Am J Physiol Gastrointest Liver Physiol. 2004 Sep;287(3):G582-91. doi: 10.1152/ajpgi.00087.2004. Epub 2004 Apr 29. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15117677 - A possible role for Ca(2+)/calmodulin-dependent protein kinase IV during pancreatic acinar stimulus-secretion coupling.
Yoshida H, Nozu F, Lankisch TO, Mitamura K, Owyang C, Tsunoda Y. Yoshida H, et al. Biochim Biophys Acta. 2000 Jun 2;1497(1):155-67. doi: 10.1016/s0167-4889(00)00051-3. Biochim Biophys Acta. 2000. PMID: 10838169
Cited by
- Molecular and cellular mechanisms of pancreatic injury.
Thrower EC, Gorelick FS, Husain SZ. Thrower EC, et al. Curr Opin Gastroenterol. 2010 Sep;26(5):484-9. doi: 10.1097/MOG.0b013e32833d119e. Curr Opin Gastroenterol. 2010. PMID: 20651589 Free PMC article. Review. - Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase Cε-dependent manner.
Wyatt TA, Sisson JH, Allen-Gipson DS, McCaskill ML, Boten JA, DeVasure JM, Bailey KL, Poole JA. Wyatt TA, et al. Am J Pathol. 2012 Aug;181(2):431-40. doi: 10.1016/j.ajpath.2012.04.022. Epub 2012 Jun 5. Am J Pathol. 2012. PMID: 22677421 Free PMC article. - Risk factors for pancreatic cancer: underlying mechanisms and potential targets.
Kolodecik T, Shugrue C, Ashat M, Thrower EC. Kolodecik T, et al. Front Physiol. 2014 Jan 16;4:415. doi: 10.3389/fphys.2013.00415. eCollection 2013. Front Physiol. 2014. PMID: 24474939 Free PMC article. Review. - PKD signaling and pancreatitis.
Yuan J, Pandol SJ. Yuan J, et al. J Gastroenterol. 2016 Jul;51(7):651-9. doi: 10.1007/s00535-016-1175-3. Epub 2016 Feb 15. J Gastroenterol. 2016. PMID: 26879861 Free PMC article. Review. - P21-activated kinase 4 in pancreatic acinar cells is activated by numerous gastrointestinal hormones/neurotransmitters and growth factors by novel signaling, and its activation stimulates secretory/growth cascades.
Ramos-Alvarez I, Jensen RT. Ramos-Alvarez I, et al. Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G302-G317. doi: 10.1152/ajpgi.00005.2018. Epub 2018 Apr 19. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29672153 Free PMC article.
References
- Cosen-Binker LI, Lam PP, Binker MG, et al. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem. 2007;282:13047–13058. - PubMed
- Satoh A, Gukovskaya AS, Nieto JM, et al. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582–591. - PubMed
- Satoh A, Gukovskaya AS, Reeve JR, Jr, et al. Ethanol Sensitizes NF-{kappa}B Activation in Pancreatic Acinar Cells through effects on Protein Kinase C Epsilon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G432–G438. - PubMed
- Bastani B, Yang L, Baldassare JJ, et al. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta. 1995;1269:307–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DK69702/DK/NIDDK NIH HHS/United States
- R21 DK069702/DK/NIDDK NIH HHS/United States
- R01 DK54021/DK/NIDDK NIH HHS/United States
- R01 DK033850/DK/NIDDK NIH HHS/United States
- R01 DK054021/DK/NIDDK NIH HHS/United States
- R01 DK33850/DK/NIDDK NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- 5 P60 AA11999/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources