Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis - PubMed (original) (raw)
Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis
Jeffrey D Johnson et al. Neuron. 2009.
Abstract
Episodic memory retrieval is thought to involve reinstatement of the neurocognitive processes engaged when an episode was encoded. Prior fMRI studies and computational models have suggested that reinstatement is limited to instances in which specific episodic details are recollected. We used multivoxel pattern-classification analyses of fMRI data to investigate how reinstatement is associated with different memory judgments, particularly those accompanied by recollection versus a feeling of familiarity (when recollection is absent). Classifiers were trained to distinguish between brain activity patterns associated with different encoding tasks and were subsequently applied to recognition-related fMRI data to determine the degree to which patterns were reinstated. Reinstatement was evident during both recollection- and familiarity-based judgments, providing clear evidence that reinstatement is not sufficient for eliciting a recollective experience. The findings are interpreted as support for a continuous, recollection-related neural signal that has been central to recent debate over the nature of recognition memory processes.
Figures
Figure 1. Behavioral Performance
(A) Mean (+SEM) proportions of responses according to the test item condition. (B) Mean (+SEM) response time (RT) data. The Other category reflects collapsed Unsure Old, Unsure New, and Sure New responses (due to low individual trial numbers). The RT data for Remember responses to new items are based on only 12 subjects contributing such responses.
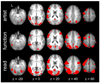
Figure 2. Importance Maps
Group mean importance maps for the three study tasks, overlaid on axial slices of the mean normalized anatomical data (coordinates in Talairach space). The colored areas depict voxels where importance values exceeded arbitrary thresholds of .001 positively (red) and -.001 negatively (green; see middle row, right-most column). L = left.
Figure 3. Classifier Accuracy
Mean classifier accuracy (+SEM) collapsed across all response categories and separated by response category. Time point (TR) 1 corresponds to test item onset. Shaded bars indicate the TRs during which classifier accuracy was significantly above chance (.33; correcting for multiple comparisons).
Figure 4. Classifier Output
Mean values (+SEM) of the classifier’s correct output node, (A) averaged over all three study tasks, and (B) over only the Artist and Function tasks. Each bar reflects classifier output for a given response category and time point (TR). Brackets indicate significant differences between responses (correcting for multiple comparisons).
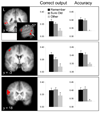
Figure 5. Equivalent Reinstatement Effects
Results of searchlight analyses where reinstatement was equivalent for test items designated with Remember and Sure Old responses (see main text for details of the contrast procedure). Histograms reflect the mean (+SEM) output values at the correct classifier node (left column) and classifier accuracy (right column; chance = .33) within the depicted clusters in lateral temporal cortex, superior frontal gyrus, and inferior frontal gyrus. All effects depicted here survived a cluster-wise threshold of p < .05 and are overlaid on the mean anatomical image (coordinates in Talairach space). L = left.
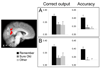
Figure 6. Selective Reinstatement Effects
Results of searchlight analyses showing selective reinstatement for test items designated with Remember responses (compared to Sure Old responses; see main text for contrast procedure). The histograms provide the mean (+SEM) output value at the correct classifier node and the mean classifier accuracy within the depicted clusters of (A) posterior cingulate and (B) retrosplenial cortex. Both effects survived a cluster-wise threshold of p < .05. See Figure 5 caption for further display details.
Similar articles
- The Effects of Age on the Neural Correlates of Recollection Success, Recollection-Related Cortical Reinstatement, and Post-Retrieval Monitoring.
Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. Wang TH, et al. Cereb Cortex. 2016 Apr;26(4):1698-1714. doi: 10.1093/cercor/bhu333. Epub 2015 Jan 28. Cereb Cortex. 2016. PMID: 25631058 Free PMC article. - Neural reinstatement and the amount of information recollected.
Leiker EK, Johnson JD. Leiker EK, et al. Brain Res. 2014 Sep 25;1582:125-38. doi: 10.1016/j.brainres.2014.07.026. Epub 2014 Jul 24. Brain Res. 2014. PMID: 25064431 - Knowledge supports memory retrieval through familiarity, not recollection.
Wang WC, Brashier NM, Wing EA, Marsh EJ, Cabeza R. Wang WC, et al. Neuropsychologia. 2018 May;113:14-21. doi: 10.1016/j.neuropsychologia.2018.01.019. Epub 2018 Jan 31. Neuropsychologia. 2018. PMID: 29391248 Free PMC article. - ROC in animals: uncovering the neural substrates of recollection and familiarity in episodic recognition memory.
Sauvage MM. Sauvage MM. Conscious Cogn. 2010 Sep;19(3):816-28. doi: 10.1016/j.concog.2010.06.023. Epub 2010 Aug 5. Conscious Cogn. 2010. PMID: 20691613 Free PMC article. Review. - Item memory, context memory and the hippocampus: fMRI evidence.
Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M. Rugg MD, et al. Neuropsychologia. 2012 Nov;50(13):3070-9. doi: 10.1016/j.neuropsychologia.2012.06.004. Epub 2012 Jun 23. Neuropsychologia. 2012. PMID: 22732490 Free PMC article. Review.
Cited by
- Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study.
Johnson JD, Suzuki M, Rugg MD. Johnson JD, et al. Front Hum Neurosci. 2013 May 23;7:219. doi: 10.3389/fnhum.2013.00219. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23734122 Free PMC article. - Multivariate cross-classification: applying machine learning techniques to characterize abstraction in neural representations.
Kaplan JT, Man K, Greening SG. Kaplan JT, et al. Front Hum Neurosci. 2015 Mar 25;9:151. doi: 10.3389/fnhum.2015.00151. eCollection 2015. Front Hum Neurosci. 2015. PMID: 25859202 Free PMC article. Review. - Representations of Complex Contexts: A Role for Hippocampus.
Dimsdale-Zucker HR, Montchal ME, Reagh ZM, Wang SF, Libby LA, Ranganath C. Dimsdale-Zucker HR, et al. J Cogn Neurosci. 2022 Dec 1;35(1):90-110. doi: 10.1162/jocn_a_01919. J Cogn Neurosci. 2022. PMID: 36166300 Free PMC article. - Memory Reactivation Predicts Resistance to Retroactive Interference: Evidence from Multivariate Classification and Pattern Similarity Analyses.
Koen JD, Rugg MD. Koen JD, et al. J Neurosci. 2016 Apr 13;36(15):4389-99. doi: 10.1523/JNEUROSCI.4099-15.2016. J Neurosci. 2016. PMID: 27076433 Free PMC article. - Listening for recollection: a multi-voxel pattern analysis of recognition memory retrieval strategies.
Quamme JR, Weiss DJ, Norman KA. Quamme JR, et al. Front Hum Neurosci. 2010 Aug 10;4:61. doi: 10.3389/fnhum.2010.00061. eCollection 2010. Front Hum Neurosci. 2010. PMID: 20740073 Free PMC article.
References
- Bishop C. Neural Networks for Pattern Recognition. New York: Oxford University Press; 1995.
- Coltheart M. The MRC psycholinguistic database. Q. J. Exp. Psychol. A. 1981;33:497–505.
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. - PubMed
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources