Exploiting synthetic lethal interactions for targeted cancer therapy - PubMed (original) (raw)
Review
Exploiting synthetic lethal interactions for targeted cancer therapy
H Christian Reinhardt et al. Cell Cycle. 2009.
Abstract
Emerging data suggests that synthetic lethal interactions between mutated oncogenes/tumor suppressor genes and molecules involved in DNA damage signaling and repair can be therapeutically exploited to preferentially kill tumor cells. In this review, we discuss the concept of synthetic lethality, and describe several recent examples in which this concept was successfully implemented to target tumor cells in culture, in mouse models, and in human cancer patients.
Figures
Figure 1
Exploiting the synthetic lethal relationship between PArP1 and BrCA1/2 for the targeted treatment of HR-deficient human tumors. HR can serve as a backup DNA repair pathway to resolve DSBs resulting from replication fork collapse. (A) In normal cells, base modifications are repaired using base excision repair prior to S-phase entry. (B) Pharmacological inhibition of the base excision repair component PARP1 results in unrepaired SSBs, which collapse replication forks into DSBs during S-phase. The newly synthesized sister chromatid can serve as a template for HR-mediated repair in BRCA1/2-proficient cells. In BRCA1/2-deficient cancer cells HR-mediated repair of PARP inhibitor-induced DSBs in not available. Instead, error-prone repair pathways, such as NHEJ and SSA are utilized, which results in progressive genomic instability and ultimately cell death.
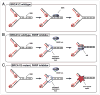
Figure 2
Exploiting synthetic lethal interactions for the treatment of human cancer. DNA damage signaling networks commonly show extensive rewiring incancercells.(A)Inp53andATM-proficient cancer cells primarily promotes apoptosis. Due to a functional proapoptotic ATM-Chk2-p53- Puma/Noxa signaling axis conventional DNA-damaging chemotherapeutics should be recommended. (B) Loss of p53 in cancer cells largely abrogates DNA damage-induced apoptosis. in these cells ATM signaling is re-directed to induce a robust cell cycle arrest following genotoxic stress. ATM-mediated homologous recombination repair remains intact in p53-deficient cancer cells. Rewiring of DNA damage-induced ATM signaling promotes cellular survival in response to DNA damage. Treatment of p53-deficient tumors should include a combination of conventional DNA-damaging chemotherapy and ATM inhibitors. (C) Loss of ATM selectively reduces the induction of the pro-apoptotic p53 target genes Puma and Noxa following genotoxic stress. induction of the cell cycle-regulatory p53 target genes p21 and _Gadd45_α remains intact allowing ATM-depleted cancer cells to arrest the cell cycle after DNA damage. ATM-deficient cancer cells with retained p53 expression depend on the DNA-PKcs-mediated NHEJ pathway to repair chemotherapy-induced DSBs and maintain genomic stability. Abolishing DNA-PKcs signaling in these cells results in a dramatically increased sensitivity to DNA-damaging chemotherapy. Treatment of ATM-deficient tumors with retained p53 expression should include a combination of conventional DNA-damaging chemotherapy and DNA-PKcs inhibitors. (D) The combined loss of ATM and p53 precludes the execution of functional cell cycle checkpoints in cancer cells that are exposed to DNA-damaging agents. This inability to halt progression through the cell cycle despite the presence of DNA damage ultimately results in mitotic catastrophe. p53 and ATM-deficient cancer cells should be exquisitely sensitive to treatment with conventional DNA-damaging chemotherapy.
Similar articles
- Inhibition of poly(ADP-ribose) polymerase (PARP) and ataxia telangiectasia mutated (ATM) on the chemosensitivity of mantle cell lymphoma to agents that induce DNA strand breaks.
Golla RM, Li M, Shen Y, Ji M, Yan Y, Fu K, Greiner TC, McKeithan TW, Chan WC. Golla RM, et al. Hematol Oncol. 2012 Dec;30(4):175-9. doi: 10.1002/hon.1020. Epub 2011 Dec 14. Hematol Oncol. 2012. PMID: 22170260 - Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents.
Haince JF, Kozlov S, Dawson VL, Dawson TM, Hendzel MJ, Lavin MF, Poirier GG. Haince JF, et al. J Biol Chem. 2007 Jun 1;282(22):16441-53. doi: 10.1074/jbc.M608406200. Epub 2007 Apr 11. J Biol Chem. 2007. PMID: 17428792 - Exploiting synthetic lethal interactions between DNA damage signaling, checkpoint control, and p53 for targeted cancer therapy.
Morandell S, Yaffe MB. Morandell S, et al. Prog Mol Biol Transl Sci. 2012;110:289-314. doi: 10.1016/B978-0-12-387665-2.00011-0. Prog Mol Biol Transl Sci. 2012. PMID: 22749150 Review. - ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency.
Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X, Li WM, Xu XL, De Soto JA, Takai H, Mai S, Elledge SJ, Motoyama N, Deng CX. Cao L, et al. EMBO J. 2006 May 17;25(10):2167-77. doi: 10.1038/sj.emboj.7601115. Epub 2006 May 4. EMBO J. 2006. PMID: 16675955 Free PMC article. - p53: guardian of the genome and policeman of the oncogenes.
Efeyan A, Serrano M. Efeyan A, et al. Cell Cycle. 2007 May 2;6(9):1006-10. doi: 10.4161/cc.6.9.4211. Epub 2007 May 28. Cell Cycle. 2007. PMID: 17457049 Review.
Cited by
- A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer.
Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, Craig-Mueller N, Colinge J, Duernberger G, Nijman SM. Muellner MK, et al. Nat Chem Biol. 2011 Sep 25;7(11):787-93. doi: 10.1038/nchembio.695. Nat Chem Biol. 2011. PMID: 21946274 Free PMC article. - The chromatin-binding domain of Ki-67 together with p53 protects human chromosomes from mitotic damage.
Garwain O, Sun X, Iyer DR, Li R, Zhu LJ, Kaufman PD. Garwain O, et al. Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2021998118. doi: 10.1073/pnas.2021998118. Proc Natl Acad Sci U S A. 2021. PMID: 34353903 Free PMC article. - Safety, Tolerability, and Pharmacokinetics of Senaparib, a Novel PARP1/2 Inhibitor, in Chinese Patients With Advanced Solid Tumors: A Phase I Trial.
Cao J, Guo H, Ji D, Shen W, Zhang S, Hsieh CY, Xiong Cai S, Edward Tian Y, Xu C, Zhang P, Xu B. Cao J, et al. Oncologist. 2023 Dec 11;28(12):e1259-e1267. doi: 10.1093/oncolo/oyad163. Oncologist. 2023. PMID: 37338150 Free PMC article. Clinical Trial. - Mitogen-activated protein kinase-activated protein kinase-2 (MK2) and its role in cell survival, inflammatory signaling, and migration in promoting cancer.
Morgan D, Berggren KL, Spiess CD, Smith HM, Tejwani A, Weir SJ, Lominska CE, Thomas SM, Gan GN. Morgan D, et al. Mol Carcinog. 2022 Feb;61(2):173-199. doi: 10.1002/mc.23348. Epub 2021 Sep 24. Mol Carcinog. 2022. PMID: 34559922 Free PMC article. - Synthetic lethality prediction in DNA damage repair, chromatin remodeling and the cell cycle using multi-omics data from cell lines and patients.
Markowska M, Budzinska MA, Coenen-Stass A, Kang S, Kizling E, Kolmus K, Koras K, Staub E, Szczurek E. Markowska M, et al. Sci Rep. 2023 Apr 29;13(1):7049. doi: 10.1038/s41598-023-34161-4. Sci Rep. 2023. PMID: 37120674 Free PMC article.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES015339/ES/NIEHS NIH HHS/United States
- R01 ES015339/ES/NIEHS NIH HHS/United States
- CA112967/CA/NCI NIH HHS/United States
- U54-CA112967-03/CA/NCI NIH HHS/United States
- P50 GM068762/GM/NIGMS NIH HHS/United States
- GM68762/GM/NIGMS NIH HHS/United States
- U54 CA112967/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous