Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice - PubMed (original) (raw)
. 2009 Oct;119(10):3059-69.
doi: 10.1172/JCI38770. Epub 2009 Sep 14.
Affiliations
- PMID: 19759515
- PMCID: PMC2752071
- DOI: 10.1172/JCI38770
Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice
Zhaoyong Hu et al. J Clin Invest. 2009 Oct.
Abstract
Muscle wasting is associated with a number of pathophysiologic conditions, including metabolic acidosis, diabetes, sepsis, and high angiotensin II levels. Under these conditions, activation of muscle protein degradation requires endogenous glucocorticoids. As the mechanism(s) underlying this dependence on glucocorticoids have not been identified, we analyzed the effects of glucocorticoids on muscle wasting in a mouse model of acute diabetes. Adrenalectomized, acutely diabetic mice given a physiologic dose of glucocorticoids exhibited decreased IRS-1-associated PI3K activity in muscle and progressive muscle atrophy. These responses were related to increased association of PI3K with the glucocorticoid receptor (GR). In mice with muscle-specific GR deletion (referred to as MGRKO mice), acute diabetes minimally suppressed IRS-1-associated PI3K activity in muscle and did not cause muscle atrophy. However, when a physiologic dose of glucocorticoids was given to mice with muscle-specific IR deletion, muscle protein degradation was accelerated. Fluorescence resonance energy transfer and an in vitro competition assay revealed that activated GRs competed for PI3K, reducing its association with IRS-1. Reexpression of WT GRs or those with a mutation in the nuclear localization signal in the muscle of MGRKO mice indicated that competition for PI3K was a prominent mechanism underlying reduced IRS-1-associated PI3K activity. This nongenomic influence of the GR contributes to activation of muscle protein degradation. We therefore conclude that stimulation of muscle proteolysis requires 2 events, increased glucocorticoid levels and impaired insulin signaling.
Figures
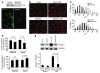
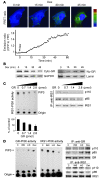
Figure 1. Glucocorticoids are necessary for diabetes-induced activation of proteolytic pathways in muscle.
(A) Muscles from control mice (C), diabetic mice (CS), and 4 groups of adrenalectomized mice were examined: AS, diabetes; ASD, diabetes plus a pathophysiologic dose of Dex; AD, with no diabetes but with the same dose of Dex; and A, ADX only (n = 6 in each group). Atrogin-1/MAFbx mRNA expression was highest in muscle of CS or ASD mice; it was not increased in AD mice, but was minimally raised in AS mice. (B) In the same groups of mice, the pattern of muscle p-Akt levels was reciprocal to that of Atrogin-1/MAFbx mRNA levels. (C) The pattern of p-FoxO1 levels in muscles was parallel to that of p-Akt levels. (D) After immunoprecipitation with anti–IRS-1 antibodies, IRS-1–associated PI3K activities in muscles of the different groups paralleled the p-Akt pattern. (E) After immunoprecipitation with anti-GR antibodies, the pattern of GR-associated PI3K activities was reciprocal to that of IRS-1–associated PI3K activities. (F) After immunoprecipitation with anti–IRS-1 or anti-GR antibodies, Western blot analyses of PI3K subunits p110 and p85 revealed the same patterns as in D and E.
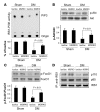
Figure 2. Muscle-specific GR knockout (MGRKO) prevented the accelerated muscle proteolysis induced by diabetes.
(A) Immunostaining of dystrophin (red) and GR (green) and Western blotting revealed minimal expression of the GR in myofibers of MGRKO mice. Original magnification, ×400. (B and C) The distribution of muscle fiber sizes in control (lox/lox) or MGRKO mice with and without acute diabetes (DM) (n = 3 in both groups and 300 myofibers examined) differed. The shift of muscle fiber sizes associated with diabetes in lox/lox mice was partially corrected in MGRKO mice. Original magnification, ×200. (D) Rates of protein synthesis and degradation in muscles of MGRKO and lox/lox mice demonstrated that the absence of the GR prevented the increase in protein degradation induced by diabetes (n = 6 in each group). Diabetes depressed protein synthesis in muscle of both MGRKO and lox/lox mice. (E) The increased expression of Atrogin-1/MAFbx in muscle of diabetic lox/lox mice was eliminated in muscle of diabetic MGRKO mice (n = 6 in both groups).
Figure 3. Muscle-specific GR knockout prevented diabetes-induced downregulation of IRS-1–associated PI3K.
(A) Suppression of IRS-1–associated PI3K activity in muscle of diabetic lox/lox mice was significantly reversed in muscle of diabetic MGRKO mice (n = 6 in each group). (B) Activities of IRS-1–associated PI3K activities (A) are paralleled by levels of p-Akt. (C) IRS-1–associated PI3K activities in muscle result in parallel levels of p-FoxO1. (D) The lower levels of IRS-1–associated p110 and p85 subunits of PI3K in muscle of diabetic lox/lox mice are partially corrected in muscle of MGRKO mice.
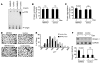
Figure 4. Both abnormal insulin signaling and increased glucocorticoid production are required to induce muscle atrophy.
(A) Muscle-specific knockout of the insulin receptor in mice (MIRKO) prevented the sharp increase in IRS-1–associated PI3K activity stimulated by insulin (representative blot from 3 separate experiments). PIP3, phosphatidylinositol. (B and C) A high physiologic dose of glucocorticoids did not change protein synthesis but stimulated protein degradation in muscle of MIRKO mice (n = 8 in each group). (D and E) MIRKO mice treated with Dex developed muscle atrophy, as indicated by a shift to smaller sizes of myofibers. Scale bar: 50 μm. (F) MIRKO mice treated with Dex exhibited increased expression of Atrogin-1/MAFbx in muscle (n = 3 in each group).
Figure 5. The increase in muscle proteolysis in MIRKO mice induced by a high physiologic dose of glucocorticoids is due to suppression of IRS-1–associated PI3K/Akt.
(A) Dex suppressed p-Akt levels, but only in muscle of MIRKO mice (n = 6 in each group of MIRKO and control mice). (B) The increase in p-Akt was associated with a significant decrease in p-FoxO1. (C and D) Dex decreased IRS-1–associated PI3K activity in muscle of MIRKO mice and increased GR-associated PI3K activity. (E and F) The changes in PI3K activity were paralleled by changes in PI3K subunit proteins p110 and p85 associated with IRS-1 or the GR.
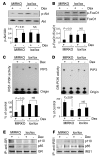
Figure 6. Identification of a nongenomic effect of the GR on muscle atrophy induced by diabetes.
(A) Left panel shows the location of the mutation that blocks the NLS1 of the GR. The right panel shows that the mutated GR (Mt-GR) does not translocate to the nucleus after addition of Dex. Original magnification, ×200. (B) Blue-green indicates electroporation-induced expression of wild-type or mutated GR in myofibers of TA muscles of MGRKO mice (n = 5 in each group). Compared with nondiabetic MGRKO mice (n = 3 mice), acute diabetes decreased the average size (cross-sectional area [CSA]) of myofibers reexpressing the wild-type or mutant GR. The graph shows that the wild-type GR induced a more severe atrophy compared with the mutated GR. Original magnification, ×200. (C) Muscles from MGRKO mice treated as in B were examined for Atrogin-1/MAFbx mRNA. The graph shows that reexpression of the wild-type GR raised mRNA levels more than the mutant GR.
Figure 7. The activated GR competes with IRS-1 for PI3K.
(A) Dex treatment of COS1 cells transfected with GR-CFP (donor) and p85-YFP (recipient) stimulated a time-dependent interaction between GR and p85, as assessed by FRET analysis (n = 3 separate experiments). The box shows the area where emission ratio was measured. (B) After addition of Dex, a substantial portion of the GR was in the cytosol (Cyto) despite accumulation of the GR in the nucleus (Nu). (C) In an in vitro competition assay, addition of increasing amounts of recombinant GR led to a progressive decrease in the PI3K activity and PI3K subunits associated with IRS-1 (n = 3). (D) C2C12 muscle cells were treated with insulin (INS), insulin plus Dex (I+D), or Dex alone. Dex alone increased GR-associated PI3K as well as PI3K subunit proteins. Cells treated with insulin alone had increased IRS-1–associated PI3K activity and minimal GR-associated PI3K activity. Treatment with insulin largely overcame the Dex-induced depression of IRS-1–associated PI3K activity.
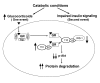
Figure 8. Catabolic conditions not only impair insulin signaling in muscle (one event) but also stimulate glucocorticoid production (second event), leading to muscle atrophy.
Glucocorticoids activate the GR in muscle, leading to a competition with IRS-1 for association of PI3K subunits p110 and p85. The association of these subunits with the GR reduces IRS-1–associated PI3K activity and p-Akt. These changes stimulate protein degradation pathways, leading to muscle atrophy.
Similar articles
- Knockout of USP19 Deubiquitinating Enzyme Prevents Muscle Wasting by Modulating Insulin and Glucocorticoid Signaling.
Coyne ES, Bedard N, Wykes L, Stretch C, Jammoul S, Li S, Zhang K, Sladek RS, Bathe OF, Jagoe RT, Posner BI, Wing SS. Coyne ES, et al. Endocrinology. 2018 Aug 1;159(8):2966-2977. doi: 10.1210/en.2018-00290. Endocrinology. 2018. PMID: 29901692 - Glucocorticoid Receptor Signaling Impairs Protein Turnover Regulation in Hypoxia-Induced Muscle Atrophy in Male Mice.
de Theije CC, Schols AMWJ, Lamers WH, Ceelen JJM, van Gorp RH, Hermans JJR, Köhler SE, Langen RCJ. de Theije CC, et al. Endocrinology. 2018 Jan 1;159(1):519-534. doi: 10.1210/en.2017-00603. Endocrinology. 2018. PMID: 29069356 - Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle.
Braun TP, Grossberg AJ, Krasnow SM, Levasseur PR, Szumowski M, Zhu XX, Maxson JE, Knoll JG, Barnes AP, Marks DL. Braun TP, et al. FASEB J. 2013 Sep;27(9):3572-82. doi: 10.1096/fj.13-230375. Epub 2013 Jun 3. FASEB J. 2013. PMID: 23733748 Free PMC article. - Glucocorticoid-induced skeletal muscle atrophy.
Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Schakman O, et al. Int J Biochem Cell Biol. 2013 Oct;45(10):2163-72. doi: 10.1016/j.biocel.2013.05.036. Epub 2013 Jun 24. Int J Biochem Cell Biol. 2013. PMID: 23806868 Review. - Muscle wasting from kidney failure-a model for catabolic conditions.
Wang XH, Mitch WE. Wang XH, et al. Int J Biochem Cell Biol. 2013 Oct;45(10):2230-8. doi: 10.1016/j.biocel.2013.06.027. Epub 2013 Jul 16. Int J Biochem Cell Biol. 2013. PMID: 23872437 Free PMC article. Review.
Cited by
- The glucocorticoid receptor acts locally to protect dystrophic muscle and heart during disease.
Oliver T, Nguyen NY, Tully CB, McCormack NM, Sun CM, Fiorillo AA, Heier CR. Oliver T, et al. Dis Model Mech. 2024 May 1;17(5):dmm050397. doi: 10.1242/dmm.050397. Epub 2024 May 21. Dis Model Mech. 2024. PMID: 38770680 Free PMC article. - β2-Adrenoceptors activation regulates muscle trophic-related genes following acute resistance exercise in mice.
Abdalla-Silva RL, Zanetti GO, Lautherbach N, Schavinski AZ, Heck LC, Gonçalves DAP, Kettelhut IC, Navegantes LCC, Silveira WA. Abdalla-Silva RL, et al. Front Physiol. 2024 Jan 22;15:1268380. doi: 10.3389/fphys.2024.1268380. eCollection 2024. Front Physiol. 2024. PMID: 38318197 Free PMC article. - ROCK1 activates mitochondrial fission leading to oxidative stress and muscle atrophy.
Si M, Yu R, Lin H, Li F, Jung S, Thomas SS, Danesh FS, Wang Y, Peng H, Hu Z. Si M, et al. bioRxiv [Preprint]. 2023 Oct 22:2023.10.22.563469. doi: 10.1101/2023.10.22.563469. bioRxiv. 2023. PMID: 37905139 Free PMC article. Preprint. - Effects of correcting metabolic acidosis on muscle mass and functionality in chronic kidney disease: a systematic review and meta-analysis.
Visser WJ, van de Braak EEM, de Mik-van Egmond AME, van der Burgh AC, de Roos NM, Jans I, van der Hoef I, Olieman JF, Hoorn EJ, Severs D. Visser WJ, et al. J Cachexia Sarcopenia Muscle. 2023 Dec;14(6):2498-2508. doi: 10.1002/jcsm.13330. Epub 2023 Sep 20. J Cachexia Sarcopenia Muscle. 2023. PMID: 37728018 Free PMC article. Review. - Nutritional Strategies to Prevent Muscle Loss and Sarcopenia in Chronic Kidney Disease: What Do We Currently Know?
Massini G, Caldiroli L, Molinari P, Carminati FMI, Castellano G, Vettoretti S. Massini G, et al. Nutrients. 2023 Jul 11;15(14):3107. doi: 10.3390/nu15143107. Nutrients. 2023. PMID: 37513525 Free PMC article. Review.
References
- Wing S.S., Goldberg A.L. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am. J. Physiol. 1993;264:E668–E676. - PubMed
- Mitch W.E., et al. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am. J. Physiol. 1999;276:C1132–C1138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DK64233/DK/NIDDK NIH HHS/United States
- R01 DK062828/DK/NIDDK NIH HHS/United States
- P50 DK064233/DK/NIDDK NIH HHS/United States
- R37 DK037175/DK/NIDDK NIH HHS/United States
- R01 DK62828/DK/NIDDK NIH HHS/United States
- R37 DK37175/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials