Optogenetic dissection of a behavioural module in the vertebrate spinal cord - PubMed (original) (raw)
Optogenetic dissection of a behavioural module in the vertebrate spinal cord
Claire Wyart et al. Nature. 2009.
Abstract
Locomotion relies on neural networks called central pattern generators (CPGs) that generate periodic motor commands for rhythmic movements. In vertebrates, the excitatory synaptic drive for inducing the spinal CPG can originate from either supraspinal glutamatergic inputs or from within the spinal cord. Here we identify a spinal input to the CPG that drives spontaneous locomotion using a combination of intersectional gene expression and optogenetics in zebrafish larvae. The photo-stimulation of one specific cell type was sufficient to induce a symmetrical tail beating sequence that mimics spontaneous slow forward swimming. This neuron is the Kolmer-Agduhr cell, which extends cilia into the central cerebrospinal-fluid-containing canal of the spinal cord and has an ipsilateral ascending axon that terminates in a series of consecutive segments. Genetically silencing Kolmer-Agduhr cells reduced the frequency of spontaneous free swimming, indicating that activity of Kolmer-Agduhr cells provides necessary tone for spontaneous forward swimming. Kolmer-Agduhr cells have been known for over 75 years, but their function has been mysterious. Our results reveal that during early development in zebrafish these cells provide a positive drive to the spinal CPG for spontaneous locomotion.
Figures
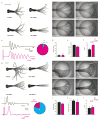
Figure 1. Optical stimulation of specific spinal neurons leads to distinct locomotor behaviors
a, Spontaneous swim (superimposed frames). b, Optical stimulation (circle) of Gal4s1020t/UAS:LiGluR evokes a “spontaneous swim”-like behavior. c, Comparison of deflection angle traces corresponding to a) (top, black) and b) (bottom, magenta, bar for stimulation) (inset: 94% of responses were a swim (n=18)). No difference in angle (p > 0.51; n=9) (d), or frequency (f), but more oscillations (e), in light-induced swims. g, Escape elicited by a water jet (trapeze) consists of sharp C-turn away from stimulus followed by a forward swim. h, Light-induced escape induced by stimulation of RB cells in Gal4s1102t/UAS:LiGluR larvae. i, Tail deflection traces corresponding to g) (top) and h) (bottom) (inset: 79% of responses were an escape (n= 11)). j-l, No difference in j) deflection angle (p > 0.13; n=7); k, frequency (p > 0.42; n=7); l, number of oscillations ((p > 0.4101;n=7).
Figure 2. The Gal4s1020t line drives expression in motoneurons and KA neurons
a, Expression in ventral cells including motoneurons projecting out of cord (arrows) (lateral view). b-e, Random labeling in Gal4s1020t/BGUG identifies solely two cell types: b, primary (top) and secondary (bottom) motoneurons. Dorsal (c) and lateral (d-f) views of neuron with a central ipsilateral ascending axon. Note contact feet (red stars in c-d) and a “toothbrush” morphology (e) (cilia, yellow arrows) characteristic of KAs. In c), larva was slightly tilted to show enlarged contacts on axon (midline, red line and segment, white lines). e, Dense BGUG pattern with multiple KAs shows the alignment of the brush of cilia (arrows) with central canal. f, The ascending axon runs near the ventral edge of the spinal cord before aiming dorsally. g,h, KAs at 5dpf in Gal4s1020t/BGUG (green) are GABAergic neurons (anti-GAD (g) and anti-GABA (h) immunostaining in red). Scale bars = 25μm.
Figure 3. Optical stimulation of KAs of Gal4s1003t line induces a forward swim
a, Expression in spinal cord is confined to cells close to the midline (dorsal view). b, Lateral view with BGUG shows ventral neurons with a central axon forming contact feet (red stars), characteristic “toothbrush” morphology (arrow) of KAs. c, d, These cells (green) are GABAergic (anti-GAD and anti-GABA staining in red). e-f, Optical stimulation induces a swim-like response. e, Superimposed images. f, Deflection angle trace. g-i, No difference in deflection angle (g), frequency (h) and number of oscillations (i) between Gal4s1003t (blue) to Gal4s1020t (magenta) (respectively p>0.85; p>0.98, p>0.36, n=9). j, Side-to-side comparison of number of oscillations evoked by a 100ms pulse of light shows that only lines expressing in KAs show a swim-like response while line with motoneurons (MNs) and no KAs (Gal4s1041t, black; Hb9:Gal4, orange), do not. k, Reduction of the spontaneous swimming frequency in Gal4s1003t/UAS:TeTxLC-CFP (p> 0.0075; n=10) but no change in the probability of touch-response (p> 0.45; n=12).
Figure 4. Dissection of the light-evoked responses in Gal4s1020t and Gal4s1102t by unilateral stimulation and lesion studies
a-c, Patterned illumination for stimulation. a, semi-restrained Gal4s1020t larva aimed bilaterally (cartoon and fluorescence image of Kaede expression in three segments, top) and on left (L) or right (R) side (bottom panels, Scale bar = 25μm). b-c, Deflection angle traces and mean values induced by L (green) and R (red) stimulation; b) L and R activations induce similar symmetric oscillations of tail in Gal4s1020t line (n=5). c, L and R activations induce large and opposite directed C-bends in Gal4s1102t (n=9). d-f, Effect induced by isolation of the spine. d, Pattern in Gal4s1020t pre (top) and post (below) lesion. e-f, No reduction of the light-induced swim behavior in Gal4s1020t ((e), n=7) but abolition of the light-induced escape behavior in Gal4s1102t ((f), n=4) (pre and post-lesion, top and bottom).
Similar articles
- Modeling spinal locomotor circuits for movements in developing zebrafish.
Roussel Y, Gaudreau SF, Kacer ER, Sengupta M, Bui TV. Roussel Y, et al. Elife. 2021 Sep 2;10:e67453. doi: 10.7554/eLife.67453. Elife. 2021. PMID: 34473059 Free PMC article. - Neural control and modulation of swimming speed in the larval zebrafish.
Severi KE, Portugues R, Marques JC, O'Malley DM, Orger MB, Engert F. Severi KE, et al. Neuron. 2014 Aug 6;83(3):692-707. doi: 10.1016/j.neuron.2014.06.032. Epub 2014 Jul 24. Neuron. 2014. PMID: 25066084 Free PMC article. - Partly shared spinal cord networks for locomotion and scratching.
Berkowitz A, Hao ZZ. Berkowitz A, et al. Integr Comp Biol. 2011 Dec;51(6):890-902. doi: 10.1093/icb/icr041. Epub 2011 Jun 22. Integr Comp Biol. 2011. PMID: 21700568 - The CPGs for Limbed Locomotion-Facts and Fiction.
Grillner S, Kozlov A. Grillner S, et al. Int J Mol Sci. 2021 May 30;22(11):5882. doi: 10.3390/ijms22115882. Int J Mol Sci. 2021. PMID: 34070932 Free PMC article. Review. - Active mechanosensory feedback during locomotion in the zebrafish spinal cord.
Knafo S, Wyart C. Knafo S, et al. Curr Opin Neurobiol. 2018 Oct;52:48-53. doi: 10.1016/j.conb.2018.04.010. Epub 2018 Apr 25. Curr Opin Neurobiol. 2018. PMID: 29704750 Review.
Cited by
- Epidural optogenetics for controlled analgesia.
Bonin RP, Wang F, Desrochers-Couture M, Ga Secka A, Boulanger ME, Côté DC, De Koninck Y. Bonin RP, et al. Mol Pain. 2016 Mar 9;12:1744806916629051. doi: 10.1177/1744806916629051. Print 2016. Mol Pain. 2016. PMID: 27030718 Free PMC article. - Silencer-delimited transgenesis: NRSE/RE1 sequences promote neural-specific transgene expression in a NRSF/REST-dependent manner.
Xie X, Mathias JR, Smith MA, Walker SL, Teng Y, Distel M, Köster RW, Sirotkin HI, Saxena MT, Mumm JS. Xie X, et al. BMC Biol. 2012 Nov 30;10:93. doi: 10.1186/1741-7007-10-93. BMC Biol. 2012. PMID: 23198762 Free PMC article. - Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits.
Bernstein JG, Garrity PA, Boyden ES. Bernstein JG, et al. Curr Opin Neurobiol. 2012 Feb;22(1):61-71. doi: 10.1016/j.conb.2011.10.023. Epub 2011 Nov 24. Curr Opin Neurobiol. 2012. PMID: 22119320 Free PMC article. Review. - Neurochemistry: Lighting up with azobenzenes.
Woolley GA. Woolley GA. Nat Chem. 2012 Jan 24;4(2):75-7. doi: 10.1038/nchem.1255. Nat Chem. 2012. PMID: 22270639 No abstract available. - Fish in the matrix: motor learning in a virtual world.
Engert F. Engert F. Front Neural Circuits. 2013 Jan 25;6:125. doi: 10.3389/fncir.2012.00125. eCollection 2012. Front Neural Circuits. 2013. PMID: 23355810 Free PMC article.
References
- Grillner Sten. Neuron. 2006;52(5):751. - PubMed
- Agduhr E. In: Cytology and cellular pathology of the nervous system. Penfield W, editor. Vol. 2. Hoeber; New York: 1932. p. 536.
- Higashijima S, Mandel G, Fetcho JR. Journal of Comparative Neurology. 2004;480(1):1. - PubMed
- Fenaux F, Corio M, Palisses R, et al. Experimental Brain Research. 1991;86(2):393. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS035549-12/NS/NINDS NIH HHS/United States
- R01 NS053358/NS/NINDS NIH HHS/United States
- R01 NS035549/NS/NINDS NIH HHS/United States
- PN2 EY018241/EY/NEI NIH HHS/United States
- 5PN2EY018241/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous