High efficiency lipid-based siRNA transfection of adipocytes in suspension - PubMed (original) (raw)
High efficiency lipid-based siRNA transfection of adipocytes in suspension
Gail Kilroy et al. PLoS One. 2009.
Abstract
Background: Fully differentiated adipocytes are considered to be refractory to introduction of siRNA via lipid-based transfection. However, large scale siRNA-based loss-of-function screening of adipocytes using either electroporation or virally-mediated transfection approaches can be prohibitively complex and expensive.
Methodology/principal findings: We present a method for introducing small interfering RNA (siRNA) into differentiated 3T3-L1 adipocytes and primary human adipocytes using an approach based on forming the siRNA/cell complex with the adipocytes in suspension rather than as an adherent monolayer, a variation of "reverse transfection".
Conclusions/significance: Transfection of adipocytes with siRNA by this method is economical, highly efficient, has a simple workflow, and allows standardization of the ratio of siRNA/cell number, making this approach well-suited for high-throughput screening of fully differentiated adipocytes.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
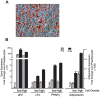
Figure 1. Adipocytes continue to differentiate after harvesting and replating post-induction.
The 3T3-L1 preadipocytes were induced to undergo adipogenesis and harvested at day 3–4 post induction when lipid droplets were clearly visible. (A) Oil Red O staining of neutral lipids forty-eight hours after harvesting and replating. (B) The gene expression of adipocyte marker genes aP2, LPL, PPARγ, and adiponectin was measured via real-time PCR upon harvesting (Pre) and forty eight hours after (Post) the adipocytes were replated at 5.4×104 cells/cm2 (low) or 1.16×105 cells/cm2 (high) and compared to the expression of each gene in preadipocytes prior to induction (preAd). Expression of each gene was assayed in triplicate, normalized to cyclophilin B gene expression, and reported as the mean and standard deviation. The results are representative of experiments carried out twice independently.
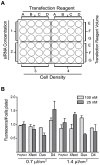
Figure 2. Optimization of siRNA transfection of adipocytes in suspension.
(A) Grid layout for testing transfection variables based on a 48 well plate format. This grid accommodates testing two concentrations of siRNA (1,2) when transfecting cells at two densities/cm2 (3,4) with four transfection reagents (A,B,C,D) at three concentrations each (E, F, G). (B) Maximal fluorescent signal/cell number plated was obtained with three transfection reagents (D4, Duo, Xfect) at either 25 nM siRNA (D4 or Xfect) or 100 nM siRNA (Duo). The siRNA is siGLO RISC-free labeled with DY-547 (rhodamine filter). The cells were plated at 1.16×105 cells/cm2. The fluorescent signals were detected and quantitated using a Flexstation 2 fluorometer (Molecular Devices) and Softmax Pro 4.8 software. The fluorescent signal was assayed in triplicate, normalized to the number of cells plated/well, and reported as the mean and standard deviation from experiments carried out twice independently.
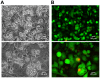
Figure 3. Adipocytes in suspension are efficiently transfected with siRNA.
Uptake of the fluorescent-labeled siRNA was assayed at 48 hours post transfection of adipocytes plated at 1.16×105 cells/cm2. (A) Brightfield and (B) fluorescent (rhodamine filter) image of the transfected cells taken with a 40X objective. (C) Co-staining with DAPI and the fluorescent-labeled siRNA indicates the siRNA is located in the cytoplasm. The image in (C) is from a different experiment than the paired images in (A) and (B). This experiment was carried out independently greater than four times.
Figure 4. 3T3-L1 adipocytes maintain viability with lipid-based siRNA transfection.
Cell viability was determined using Calcein-AM (green, viable cells) and propidium iodide (red, non-viable cells) staining in a “live-dead” assay. The assay was carried out at 48 hours post-transfection using non-targeting siRNA (Dharmacon siRNA pool #2 containing luciferase siRNA). (A) Brightfield images taken with a 20X (upper panel) and 40X objective (lower panel). (B) Fluorescent images taken with a 20X (upper panel) and 40X objective (lower panel) showing minimal PI staining. The experiment was carried out twice independently.
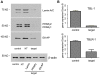
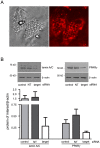
Figure 5. Specific genes are efficiently targeted with lipid-based siRNA transfection of adipocytes.
Knockdown of specific genes was assayed at 48 hours post-transfection using either the RISC-free control siRNA (control), nontargeting siRNA (NT), or siRNA targeted to the indicated gene (target). (A) Thirty-five µg of protein was loaded in each lane and separated by SDS-PAGE. Knockdown of lamin A/C, PPARγ1 and PPARγ2, and E6-AP was assayed via western blot analysis. Equal loading of each lane was determined using β-actin expression. (B) knockdown of TBL-1 and TBLR-1 was assayed via real-time PCR in triplicate and reported as the mean and standard deviation. These experiments were carried out independently greater than four times.
Figure 6. Transfection of adherent 3T3-L1 adipocytes with siRNA does not decrease expression of targeted proteins.
Forty-eight hours post-transfection, transfection efficiency was assayed as uptake of fluorescent-labeled siRNA and the siRNA-mediated effect on targeted protein expression. (A) Brightfield and fluorescent (rhodamine filter) images of the transfected cells were taken with a 40X objective. (B) Knockdown of lamin A/C and PPARγ was assayed via western blot analysis post-transfection with either the RISC-free control siRNA (control), nontargeting siRNA (NT), or siRNA targeted to the indicated gene (target). Thirty-five µg of protein was loaded in each lane and separated by SDS-PAGE. Equal loading of each lane was determined using β-actin expression. The experiment was carried out twice independently.
Figure 7. Transfection of human primary adipocytes in suspension with siRNA is associated with decreased expression of targeted proteins.
The human adipocytes were transfected on day 9 post-induction (1.165×105 cells/100 nM siRNA ) and plated at 4.1×104 cells/cm2. Forty-eight hours post-transfection, transfection efficiency was assayed as uptake of fluorescent-labeled siRNA and the siRNA-mediated effect on targeted protein expression. (A) Brightfield and fluorescent (rhodamine filter) images of the transfected cells were taken with a 40X objective. (B) Knockdown of lamin A/C and PPARγ was assayed via western blot analysis post-transfection with either the RISC-free control siRNA (control), nontargeting siRNA (NT), or siRNA targeted to the indicated gene (target). Thirty-five µg of protein was loaded in each lane and separated by SDS-PAGE. Equal loading of each lane was determined using β-actin expression. The mean and standard deviation of the ratio lamin A/C and PPARγ compared to β-actin was determined after the expression levels of each protein were quantified using Un-Scan-It software (version 6.1) from samples run in triplicate. The experiment was carried out twice independently.
Similar articles
- Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes.
Liao W, Nguyen MT, Imamura T, Singer O, Verma IM, Olefsky JM. Liao W, et al. Endocrinology. 2006 May;147(5):2245-52. doi: 10.1210/en.2005-1638. Epub 2006 Feb 23. Endocrinology. 2006. PMID: 16497797 - RNA interference of PPARgamma using fiber-modified adenovirus vector efficiently suppresses preadipocyte-to-adipocyte differentiation in 3T3-L1 cells.
Hosono T, Mizuguchi H, Katayama K, Koizumi N, Kawabata K, Yamaguchi T, Nakagawa S, Watanabe Y, Mayumi T, Hayakawa T. Hosono T, et al. Gene. 2005 Mar 28;348:157-65. doi: 10.1016/j.gene.2005.01.005. Gene. 2005. PMID: 15777692 - RNA interference-based silencing reveals the regulatory role of fatty acid-binding protein 4 in the production of IL-6 and vascular endothelial growth factor in 3T3-L1 adipocytes.
Kajimoto K, Takayanagi S, Sasaki S, Akita H, Harashima H. Kajimoto K, et al. Endocrinology. 2012 Nov;153(11):5629-36. doi: 10.1210/en.2012-1456. Epub 2012 Sep 24. Endocrinology. 2012. PMID: 23008513 - Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes.
Su X, Mancuso DJ, Bickel PE, Jenkins CM, Gross RW. Su X, et al. J Biol Chem. 2004 May 21;279(21):21740-8. doi: 10.1074/jbc.M314166200. Epub 2004 Mar 15. J Biol Chem. 2004. PMID: 15024020 - Research Progress of the Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes.
Liu X, Liu XN, Li TJ, Liu B. Liu X, et al. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017 Oct 30;39(5):727-731. doi: 10.3881/j.issn.1000-503X.2017.05.021. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017. PMID: 29125119 Review. English.
Cited by
- Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes.
Habtemichael EN, Li DT, Alcázar-Román A, Westergaard XO, Li M, Petersen MC, Li H, DeVries SG, Li E, Julca-Zevallos O, Wolenski JS, Bogan JS. Habtemichael EN, et al. J Biol Chem. 2018 Jul 6;293(27):10466-10486. doi: 10.1074/jbc.RA118.003021. Epub 2018 May 17. J Biol Chem. 2018. PMID: 29773651 Free PMC article. - Mice subjected to aP2-Cre mediated ablation of microsomal triglyceride transfer protein are resistant to high fat diet induced obesity.
Bakillah A, Hussain MM. Bakillah A, et al. Nutr Metab (Lond). 2016 Jan 8;13:1. doi: 10.1186/s12986-016-0061-6. eCollection 2016. Nutr Metab (Lond). 2016. PMID: 26752997 Free PMC article. - Regulated in development and DNA damage responses -1 (REDD1) protein contributes to insulin signaling pathway in adipocytes.
Regazzetti C, Dumas K, Le Marchand-Brustel Y, Peraldi P, Tanti JF, Giorgetti-Peraldi S. Regazzetti C, et al. PLoS One. 2012;7(12):e52154. doi: 10.1371/journal.pone.0052154. Epub 2012 Dec 18. PLoS One. 2012. PMID: 23272222 Free PMC article. - Coregulator cell cycle and apoptosis regulator 1 (CCAR1) positively regulates adipocyte differentiation through the glucocorticoid signaling pathway.
Ou CY, Chen TC, Lee JV, Wang JC, Stallcup MR. Ou CY, et al. J Biol Chem. 2014 Jun 13;289(24):17078-86. doi: 10.1074/jbc.M114.548081. Epub 2014 May 8. J Biol Chem. 2014. PMID: 24811171 Free PMC article. - A high-content endogenous GLUT4 trafficking assay reveals new aspects of adipocyte biology.
Diaz-Vegas A, Norris DM, Jall-Rogg S, Cooke KC, Conway OJ, Shun-Shion AS, Duan X, Potter M, van Gerwen J, Baird HJ, Humphrey SJ, James DE, Fazakerley DJ, Burchfield JG. Diaz-Vegas A, et al. Life Sci Alliance. 2022 Oct 25;6(1):e202201585. doi: 10.26508/lsa.202201585. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36283703 Free PMC article.
References
- Puri V, Chakladar A, Virbasius JV, Konda S, Powelka AM, et al. RNAi-based gene silencing in primary mouse and human adipose tissues. J Lipid Res. 2007;48:465–471. - PubMed
- Orlicky DJ, Schaack J. Adenovirus transduction of 3T3-L1 cells. J Lipid Res. 2001;42:460–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR021945/RR/NCRR NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- P20 RR02195/RR/NCRR NIH HHS/United States
- 1 P30-DK072476/DK/NIDDK NIH HHS/United States
- P20-RR021945/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources