Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc - PubMed (original) (raw)
Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc
Nimet Maherali et al. Curr Biol. 2009.
Abstract
Ectopic expression of Oct4, Sox2, cMyc, and Klf4 confers a pluripotent state upon several differentiated cell types, generating induced pluripotent stem cells (iPSCs) [1-8]. iPSC derivation is highly inefficient, and the underlying mechanisms are largely unknown. This low efficiency suggests the existence of additional cooperative factors whose identification is critical for understanding reprogramming. In addition, the therapeutic use of iPSCs relies on the development of efficient nongenetic means of factor delivery, and although a handful of replacement molecules have been identified, their use yields a further reduction to the already low reprogramming efficiency [9-11]. Thus, the identification of compounds that enhance rather than solely replace the function of the reprogramming factors will be of great use. Here, we demonstrate that inhibition of Tgfbbeta signaling cooperates in the reprogramming of murine fibroblasts by enabling faster, more efficient induction of iPSCs, whereas activation of Tgfbeta signaling blocks reprogramming. In addition to exhibiting a strong cooperative effect, the Tgfbeta receptor inhibitor bypasses the requirement for exogenous cMyc or Sox2, highlighting its dual role as a cooperative and replacement factor. The identification of a highly characterized pathway operating in iPSC induction will open new avenues for mechanistic dissection of the reprogramming process.
Figures
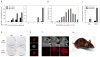
Figure 1. The Alk5 inhibitor acts cooperatively to promote iPSC induction
A. Alkaline phosphatase stain for primary iPSC colonies, treated with or without the Alk5 inhibitor (1μM) during dox induction. MEFs were infected with four factors and colonies were stained on day 12 (8d +dox/4d –dox). B. Kinetics of reprogramming in MEFs infected with four factors. Dox was applied for either 3, 4, or 5 days, with or without the Alk5 inhibitor (2μM). Colonies were counted on day 16 based on morphology; all picked clones were capable of generating dox-independent lines. C. Pluripotency of iPSCs derived after four days of dox induction using the Alk5 inhibitor. (i-iii) Immunostaining for pluripotency markers, (i) Nanog, (ii) Oct4, and (iii) Sox2. Colonies were stained after three passages without dox, thus reflecting endogenous expression. (iv-vi) Teratoma formation, demonstrating differentiation into lineages from all three germ layers: (iv) neural tissue, (v) cartilage, (vi) gut-like epithelium. D. Dose-response curve for the Alk5 inhibitor in MEFs induced with four factors. Dox and the inhibitor were applied for 8 days; colonies were quantified on day 12 based on Oct4 immunostaining. E. Effect of Tgfβ ligands in reprogramming, using four-factor infected MEFs (dox for 12d; counts on day 16 based on Oct4 immunostaining). Tgfβ concentrations: low, 1ng/mL; medium, 2.5ng/mL; high, 5ng/mL. F. Timing of Alk5 inhibitor action. The Alk5 inhibitor (1μM) was applied in 4-day time intervals. Control = no inhibitor, Full-time = inhibitor added days 1-16. Dox was withdrawn on day 12, and colonies were quantified on day 16 based on Oct4 immunostaining. G. Priming effect of the Alk5 inhibitor. Inhibitor (1μM) was applied for either 3 days before (-3 to 0d) or during the first 3 days of dox induction (0 to +3d), or both (-3d to +3d). Control = no inhibitor. Dox was withdrawn on day 8 and colonies were quantified on day 12 by Oct4 immunostaining.
Figure 2. The Alk5 inhibitor replaces the individual roles of cMyc and Sox2
A. Kinetics of reprogramming in two secondary MEF lines. Dox was applied for either 4, 6, 8, or 10 days, with or without the Alk5 inhibitor (2μM). Colonies were quantified on day 16 based on morphology and dox independence. B. Dose-response curve for the Alk5 inhibitor in secondary STEMCCA MEFs. Dox and the inhibitor were applied for 8 days, and colonies were quantified on day 12 based on Oct4 immunostaining. C. Replacement of the role of cMyc with the Alk5 inhibitor (1μM). MEFs were infected with either three (OSK) or four (OSMK) factors. Dox was withdrawn on day 8 and colonies were quantified on day 12 based on morphology and dox independence. D. Replacement of the role of Sox2 with the Alk5 inhibitor (1μM). MEFs were infected with three (OMK) or two (OK) factors and induced with dox for 16 days, then split on day 18 into media without dox. Image depicts alkaline phosphatase stain of passage 1 cultures. E. Pluripotency marker expression in an iPSC line made with the Alk5 inhibitor in the absence of Sox2. Colonies were analyzed after three passages without dox. (i) Nanog, (ii) Oct4, (iii), Sox2. F. Chimeric mice generated with OMK+inhibitor iPSC lines. iPSCs were labeled with lentivirally-delivered tdTomato, injected into diploid blastocysts, and harvested at E16.5. Three embryos are shown at identical exposures, demonstrating varying degrees of chimerism. G. Adult chimera (6 weeks) derived from an OMK+inhibitor iPSC line. Agouti coat color represents iPSC-derived cells.
Similar articles
- Small RNA-mediated regulation of iPS cell generation.
Li Z, Yang CS, Nakashima K, Rana TM. Li Z, et al. EMBO J. 2011 Mar 2;30(5):823-34. doi: 10.1038/emboj.2011.2. Epub 2011 Feb 1. EMBO J. 2011. PMID: 21285944 Free PMC article. - Two-phase analysis of molecular pathways underlying induced pluripotent stem cell induction.
Lin Z, Perez P, Lei D, Xu J, Gao X, Bao J. Lin Z, et al. Stem Cells. 2011 Dec;29(12):1963-74. doi: 10.1002/stem.752. Stem Cells. 2011. PMID: 21956995 - CXCR2 Ligands and mTOR Activation Enhance Reprogramming of Human Somatic Cells to Pluripotent Stem Cells.
Lee SJ, Kang KW, Kim JH, Lee BH, Jung JH, Park Y, Hong SC, Kim BS. Lee SJ, et al. Stem Cells Dev. 2020 Feb 1;29(3):119-132. doi: 10.1089/scd.2019.0188. Epub 2020 Jan 6. Stem Cells Dev. 2020. PMID: 31808362 - Mechanism of Induction: Induced Pluripotent Stem Cells (iPSCs).
Singh VK, Kumar N, Kalsan M, Saini A, Chandra R. Singh VK, et al. J Stem Cells. 2015;10(1):43-62. J Stem Cells. 2015. PMID: 26665937 Review. - OCT4 and SOX2 Work as Transcriptional Activators in Reprogramming Human Fibroblasts.
Narayan S, Bryant G, Shah S, Berrozpe G, Ptashne M. Narayan S, et al. Cell Rep. 2017 Aug 15;20(7):1585-1596. doi: 10.1016/j.celrep.2017.07.071. Cell Rep. 2017. PMID: 28813671 Free PMC article.
Cited by
- Increased reprogramming of human fetal hepatocytes compared with adult hepatocytes in feeder-free conditions.
Hansel MC, Gramignoli R, Blake W, Davila J, Skvorak K, Dorko K, Tahan V, Lee BR, Tafaleng E, Guzman-Lepe J, Soto-Gutierrez A, Fox IJ, Strom SC. Hansel MC, et al. Cell Transplant. 2014 Jan;23(1):27-38. doi: 10.3727/096368912X662453. Epub 2013 Feb 4. Cell Transplant. 2014. PMID: 23394081 Free PMC article. - Experimental and therapeutic opportunities for stem cells in multiple sclerosis.
Patani R, Chandran S. Patani R, et al. Int J Mol Sci. 2012 Nov 8;13(11):14470-91. doi: 10.3390/ijms131114470. Int J Mol Sci. 2012. PMID: 23203076 Free PMC article. Review. - Generation of a drug-inducible reporter system to study cell reprogramming in human cells.
Ruiz S, Panopoulos AD, Montserrat N, Multon MC, Daury A, Rocher C, Spanakis E, Batchelder EM, Orsini C, Deleuze JF, Izpisua Belmonte JC. Ruiz S, et al. J Biol Chem. 2012 Nov 23;287(48):40767-78. doi: 10.1074/jbc.M112.384024. Epub 2012 Sep 27. J Biol Chem. 2012. PMID: 23019325 Free PMC article. - Controlling destiny through chemistry: small-molecule regulators of cell fate.
Firestone AJ, Chen JK. Firestone AJ, et al. ACS Chem Biol. 2010 Jan 15;5(1):15-34. doi: 10.1021/cb900249y. ACS Chem Biol. 2010. PMID: 20000447 Free PMC article. Review. - Induced pluripotency: history, mechanisms, and applications.
Stadtfeld M, Hochedlinger K. Stadtfeld M, et al. Genes Dev. 2010 Oct 15;24(20):2239-63. doi: 10.1101/gad.1963910. Genes Dev. 2010. PMID: 20952534 Free PMC article. Review.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
- Feng B, Jiang J, Kraus P, Ng JH, Heng JC, Chan YS, Yaw LP, Zhang W, Loh YH, Han J, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11:197–203. - PubMed
- Eminli S, Utikal JS, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of Neural Progenitor Cells into iPS Cells in the Absence of Exogenous Sox2 Expression. Stem Cells 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources