The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2 - PubMed (original) (raw)
The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2
Ye Yan et al. Cell. 2009.
Abstract
The six-transmembrane protein GDE2 controls the onset and progression of spinal motor neuron differentiation through extracellular glycerophosphodiester phosphodiesterase metabolism. Although this process is likely to be tightly regulated, the relevant mechanisms that modulate its activity are unknown. Here we show that the antioxidant scavenger peroxiredoxin1 (Prdx1) interacts with GDE2, and that loss of Prdx1 causes motor neuron deficits analogous to GDE2 ablation. Prdx1 cooperates with GDE2 to drive motor neuron differentiation, and this synergy requires Prdx1 thiol-dependent catalysis. Prdx1 activates GDE2 through reduction of an intramolecular disulfide bond that bridges its intracellular N- and C-terminal domains. GDE2 variants incapable of disulfide bond formation acquire independence from Prdx1 and are potent inducers of motor neuron differentiation. These findings define Prdx1 as a pivotal regulator of GDE2 activity and suggest roles for coupled thiol-redox-dependent cascades in controlling neuronal differentiation in the spinal cord.
Figures
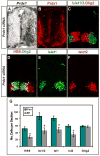
Figure 1. Prdx1 interacts and overlaps with GDE2 in the ventral spinal cord
(A) Silver stained SDS-PAGE gel shows a 23KDa band (arrow) coIPs with GDE2. NLZ, control plasmid; GDE2, pCAGGS-GDE2NLZ. (B, E) Tryptic peptides (red) from LC-MS/MS analyses of proteins derived from the 23KDa band correspond to human (B) and chick Prdx1 (E). (C, D, F) In situ hybridization of Gde2 and Prdx1 mRNA, and immunohistochemical analyses on transverse sections of St 19–20 chick spinal cords. Prdx1 and GDE2 proteins colocalize in intermediate (IZ) and marginal zone (MZ) cells but not in ventricular zone progenitors (VZ). (G) CoIPs of transfected HEK293T cells show HA-tagged Prdx1 interacts with the N-terminus of GDE2.
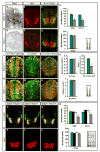
Figure 2. Prdx1 is required for motor neuron generation
(A) In situ hybridization and immunohistochemical analyses (B–F) of transverse sections of St 20–21 chick spinal cords electroporated with Prdx1 siRNAs (left). HB9+ motor neurons are visualized in red in D. (G) Graph shows reduction of postmitotic motor neurons on Prdx1 silencing. Con= nonelectroporated side, EP= electroporated side (mean ± s.e.m.) * = HB9, p=0.000004; Islet1/2, p= 0.00001; Isl1, p=0.00009; Isl2, p= 0.00008; Olig2, p= 0.2; paired student’s t-test, n= 6.
Figure 3. _Prdx1_−/− embryos show deficits in motor neuron generation
(A, E) In situ hybridization and (B, C, F, G, H–J, L–N) immunohistochemical analyses of transverse sections of mouse E9.5 Prdx1+/+ and Prdx1 null spinal cords. (D) Graphs show motor neuron loss in Prdx1 nulls (mean ± s.e.m.) * HB9 p= 0.00054, * Isl1/2 p= 0.0004; Olig2 p= 0.69; two-tailed student’s t-test, n= 5. (K) Graphs show deficits in cell-cycle exit in Prdx1 nulls (mean ± s.e.m.) * = cell cycle exit, p= 0.0002, S-phase p= 0.63, M phase p= 0.8; two-tailed student’s t-test, n= 5. (O-Q, S-U) Immunohistochemical analyses of transverse sections of mouse E9.5 spinal cords. (R) Graphs show motor neuron loss in double heterozygotes for Prdx1 and Gde2 (mean ± s.e.m.) 2 way ANOVA shows significant interaction between GDE2 and Prdx1; *HB9 p= 0.00783, * Isl1/2 p= 6.54×10−5, Olig2 p= 0.72; n= 5.
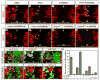
Figure 4. Prdx1/GDE2 complexes drive motor neuron differentiation in vivo
(A–N) Immunohistochemical analyses of transverse sections of ventral St 19–20 chick spinal cords electroporated (left) with GDE2 and Prdx1. Arrowheads mark terminally differentiated neurons in the ventricular zone (VZ). The VZ was defined by BrdU pulse labeling to mark S-phase cells at lateral margins. Midline: vertical arrow, VZ: horizontal arrow. (M, N) Same section stained with HA and LacZ to detect Prdx1 and GDE2 respectively (O) Graph quantifying ectopic motor neurons in the VZ of electroporated embryos (mean ± s.e.m; n= 5–10). Prdx1 overexpression weakly induces ectopic motor neurons, presumably through interactions with GDE2 related proteins in VZ cells such as GDE6 (C.H. Lee and S. Sockanathan; unpublished observations).
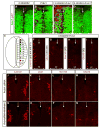
Figure 5. GDE2 and Prdx1 regulate spinal interneuron differentiation
(A–D; F–Q) Immunohistochemical analysis of transverse sections of St 20–21 dorsal chick spinal cords, electroporated on the left. Arrows mark the midline. VZ: ventricular zone, VZ extent = double headed arrows. Prdx1*=Prdx1C52.173.S. (E) Schematic of the spinal cord showing the location of dorsal (dI1-6) and ventral (V0-V3) interneurons in relation to motor neurons (MN). (F-I) Prdx1 and GDE2 overexpression induces multiple interneuron subtypes in spinal progenitors [defined by their molecular gene expression profiles; Helms and Johnson, (2003)] within their respective dorsal-ventral domains. (J-M) Ablation of Prdx1 by electroporation of siRNA reduces the number of dorsal and ventral spinal interneurons. (N-Q) Spinal interneuron populations are rescued by electroporation of hPrdx1, which is insensitive to the electroporated Prdx1 siRNA.
Figure 6. Prdx1 reduces an intracellular disulfide bridge in GDE2
(A) Western blots of AMS modified surface biotinylated GDE2 in transfected HEK293T cells. Red dots mark horizontal position of the bands. Similar results were obtained with total protein. Addition of DTT caused further shifts in GDE2 mobility consistent with the presence of additional disulfide bonds in GDE2. MS analysis reveals that C15 and C18 can form a disulfide bond that is insensitive to Prdx1 activity (data not shown) (B) Schematic of intracellular cysteines in GDE2, other cysteines are not marked (C) Western blots of IP-ed GDE2 detecting biotinylated GDE2 compared with GDE2. Graphs quantify the amount of biotinylated GDE2 normalized to total GDE2, mean ± s.e.m., GDE2 + GFP values are normalized to 1, GDE2 + Prdx1 *p= 6.4×10−18, GDE2C15.18S + Prdx1 *p= 2.05×10−13, GDE2C25.576S + Prdx1 p= 0.27; n= 7 assays; one sample student’s t-test. (D) Non-reducing gels of GDE2 coIPs from transfected HEK293T cells with or without β-mercaptoethanol (β-ME). Arrow = 120–130KDa mixed-disulfide Prdx1/glycosylated GDE2 intermediate. Black circles: non-specific bands; open circles: higher order Prdx1-containing oligomers, Prdx1 monomers. The band comigrating with the Prdx1 monomer in lane 1 is not specific and forms a minor component when Prdx1 complexes interacting with GDE2 are reduced by β-ME. (E) Relative signal intensities of GDE2 C-terminal peptides containing C576 labeled with IAM (C; oxidized thiol) or NEM (C#; free thiol) in the absence or presence of Prdx1. Ratio= NEM/IAM.
Figure 7. GDE2 variants incapable of forming the N-C disulfide bridge are more active and lose Prdx1 synergy
(A-F) Immunohistochemical analyses of ventral and dorsal (I-P) St 19–20 electroporated chick spinal cords (left). Arrowheads mark Islet2+ motor neurons in the ventricular zone (VZ). Midline: vertical arrow, VZ: horizontal arrow. (G) Graphs quantifying the percentage of ectopic motor neurons relative to the number of transfected cells (LacZ) and the number of ectopic motor neurons (H) in the VZ (mean ± s.e.m; * = p<0.001, two-tailed student’s t-test, n= 7–10; n.s.= p > 0.5). (Q) Model for Prdx1 regulation of GDE2-dependent neuronal differentiation. Reduced forms of Prdx1 bind to the N-terminus intracellular domain of GDE2. The redox active Cys of Prdx1 forms a mixed-disulfide intermediate with GDE2. Here, C576 of GDE2 participates in a direct thiol-disulfide exchange, but C25 might be utilized instead. Reduction of the GDE2 C25-C576 disulfide bond by Prdx1 activates GDE2, promoting differentiation via extracellular GDPD activity. Prdx1 is oxidized during the reaction. The reduced form of Prdx1 may be a transient component of GDE2/Prdx1 complexes.
Comment in
- Reducing the mystery of neuronal differentiation.
Novitch BG, Butler SJ. Novitch BG, et al. Cell. 2009 Sep 18;138(6):1062-4. doi: 10.1016/j.cell.2009.09.001. Cell. 2009. PMID: 19766560
Similar articles
- Prdx4 is a compartment-specific H2O2 sensor that regulates neurogenesis by controlling surface expression of GDE2.
Yan Y, Wladyka C, Fujii J, Sockanathan S. Yan Y, et al. Nat Commun. 2015 May 6;6:7006. doi: 10.1038/ncomms8006. Nat Commun. 2015. PMID: 25943695 Free PMC article. - GDE2 is essential for neuronal survival in the postnatal mammalian spinal cord.
Cave C, Park S, Rodriguez M, Nakamura M, Hoke A, Pletnikov M, Sockanathan S. Cave C, et al. Mol Neurodegener. 2017 Jan 19;12(1):8. doi: 10.1186/s13024-017-0148-1. Mol Neurodegener. 2017. PMID: 28103900 Free PMC article. - Transmembrane protein GDE2 induces motor neuron differentiation in vivo.
Rao M, Sockanathan S. Rao M, et al. Science. 2005 Sep 30;309(5744):2212-5. doi: 10.1126/science.1117156. Science. 2005. PMID: 16195461 - Peroxiredoxin 1 - an antioxidant enzyme in cancer.
Ding C, Fan X, Wu G. Ding C, et al. J Cell Mol Med. 2017 Jan;21(1):193-202. doi: 10.1111/jcmm.12955. Epub 2016 Sep 21. J Cell Mol Med. 2017. PMID: 27653015 Free PMC article. Review. - Mammalian glycerophosphodiester phosphodiesterases.
Yanaka N. Yanaka N. Biosci Biotechnol Biochem. 2007 Aug;71(8):1811-8. doi: 10.1271/bbb.70062. Epub 2007 Aug 7. Biosci Biotechnol Biochem. 2007. PMID: 17690467 Review.
Cited by
- Peroxiredoxins wear many hats: Factors that fashion their peroxide sensing personalities.
Bolduc J, Koruza K, Luo T, Malo Pueyo J, Vo TN, Ezeriņa D, Messens J. Bolduc J, et al. Redox Biol. 2021 Jun;42:101959. doi: 10.1016/j.redox.2021.101959. Epub 2021 Apr 20. Redox Biol. 2021. PMID: 33895094 Free PMC article. Review. - The glycerophospho metabolome and its influence on amino acid homeostasis revealed by brain metabolomics of GDE1(-/-) mice.
Kopp F, Komatsu T, Nomura DK, Trauger SA, Thomas JR, Siuzdak G, Simon GM, Cravatt BF. Kopp F, et al. Chem Biol. 2010 Aug 27;17(8):831-40. doi: 10.1016/j.chembiol.2010.06.009. Chem Biol. 2010. PMID: 20797612 Free PMC article. - Exposure of the SH-SY5Y Human Neuroblastoma Cells to 50-Hz Magnetic Field: Comparison Between Two-Dimensional (2D) and Three-Dimensional (3D) In Vitro Cultures.
Consales C, Butera A, Merla C, Pasquali E, Lopresto V, Pinto R, Pierdomenico M, Mancuso M, Marino C, Benassi B. Consales C, et al. Mol Neurobiol. 2021 Apr;58(4):1634-1649. doi: 10.1007/s12035-020-02192-x. Epub 2020 Nov 24. Mol Neurobiol. 2021. PMID: 33230715 Free PMC article. - Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides.
Rhee SG, Woo HA, Kil IS, Bae SH. Rhee SG, et al. J Biol Chem. 2012 Feb 10;287(7):4403-10. doi: 10.1074/jbc.R111.283432. Epub 2011 Dec 6. J Biol Chem. 2012. PMID: 22147704 Free PMC article. Review. - GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK.
Park S, Lee C, Sabharwal P, Zhang M, Meyers CL, Sockanathan S. Park S, et al. Science. 2013 Jan 18;339(6117):324-8. doi: 10.1126/science.1231921. Science. 2013. PMID: 23329048 Free PMC article.
References
- Arnér ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. - PubMed
- Bertram JS. The molecular biology of cancer. Mol Aspects Med. 2000;21:167–223. - PubMed
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine Redox Sensor in PKGIa Enables Oxidant-Induced Activation. Science. 2007;317:1393–1397. - PubMed
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. - PubMed
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS046336-05A1/NS/NINDS NIH HHS/United States
- R56 NS046336-05/NS/NINDS NIH HHS/United States
- R01 NS046336/NS/NINDS NIH HHS/United States
- R56 NS046336/NS/NINDS NIH HHS/United States
- R01 NS046336-06/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous