Regulation of pathogenesis and immunity in helminth infections - PubMed (original) (raw)
Review
. 2009 Sep 28;206(10):2059-66.
doi: 10.1084/jem.20091903. Epub 2009 Sep 21.
Affiliations
- PMID: 19770272
- PMCID: PMC2757871
- DOI: 10.1084/jem.20091903
Review
Regulation of pathogenesis and immunity in helminth infections
Rick M Maizels et al. J Exp Med. 2009.
Abstract
Helminths are multicellular eukaryotic parasites that infect over one quarter of the world's population. Through coevolution with the human immune system, these organisms have learned to exploit immunoregulatory pathways, resulting in asymptomatic tolerance of infections in many individuals. When infections and the resulting immune responses become dysregulated, however, acute and chronic pathologies often develop. A recent international meeting focused on how these parasites modulate host immunity and how control of parasitic and immunopathological disease might be achieved.
Figures
The intestinal helminth H. polygyrus. IMAGE COURTESY OF CONSTANCE FINNEY.
Figure 1.
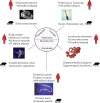
Helminth infections. A summary of the major helminth infections of humans and their mouse model counterparts. Estimates of human infection numbers are taken from Hotez et al. (2008). Note that the schematic does not include highly prevalent helminth infections such as Ascaris lumbricoides (807 million estimated infections), other important human parasites, or the prominent veterinary helminth organisms.
Figure 2.
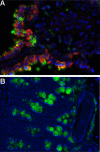
Expression of RELM proteins after exposure to helminth parasites. RELMα (green) is expressed by mannose receptor–positive cells (red) in the lungs of C57BL/6 mice challenged i.v. with S. mansoni eggs (A), and RELMβ is expressed by goblet cells of the gastrointestinal tract in C57BL/6 mice infected orally with T. muris eggs (B). Image courtesy of Meera Nair and David Artis, University of Pennsylvania.
Figure 3.
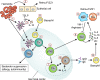
Cellular interactions in the immune response to helminths. Helminths are complex eukaryotic pathogens with multiple life stages that can affect different tissues within the parasitized host. Helminth-derived antigens can drain directly to secondary lymphoid organs or interact with cells at the site of infection, including DCs (1) or epithelial cells (2). Many helminth antigens inhibit the production of proinflammatory cytokines by DCs (3), and trigger the production of cytokines such as TSLP from epithelial cells, which inhibits IL-12 production by DCs. Antigen-carrying DCs activate naive Th cells (4), which proliferate and differentiate into precursor Th2 cells (5). pTh2 cells can then become Th2 cells (6) or Tfh cells (7). Th2 cells release various cytokines, including IL-5, which drives eosinophilia (8), and IL-4/-13, which stimulate AAMs (9). AAMs in turn produce molecules such as arginase-1 and YM-1, which dampen Th2 responses. Tfh produce IL-4 and provide help to B cells for IgG1 and IgE production (10). During the early response to helminth infections, MHC class II–expressing basophils (11) enter the reactive secondary lymphoid organs and may help to polarize the Th2 response. IgE can then activate basophils (12), which in turn produce cytokines that activate alternative macrophages. Helminth infections can also promote the development of T reg cell responses (13).
Similar articles
- Helminth parasites and immune regulation.
Gazzinelli-Guimaraes PH, Nutman TB. Gazzinelli-Guimaraes PH, et al. F1000Res. 2018 Oct 23;7:F1000 Faculty Rev-1685. doi: 10.12688/f1000research.15596.1. eCollection 2018. F1000Res. 2018. PMID: 30416709 Free PMC article. Review. - Mapping immune response profiles: the emerging scenario from helminth immunology.
Díaz A, Allen JE. Díaz A, et al. Eur J Immunol. 2007 Dec;37(12):3319-26. doi: 10.1002/eji.200737765. Eur J Immunol. 2007. PMID: 18000958 Review. - Helminth infections and host immune regulation.
McSorley HJ, Maizels RM. McSorley HJ, et al. Clin Microbiol Rev. 2012 Oct;25(4):585-608. doi: 10.1128/CMR.05040-11. Clin Microbiol Rev. 2012. PMID: 23034321 Free PMC article. Review. - Regulatory T-cells in helminth infection: induction, function and therapeutic potential.
White MPJ, McManus CM, Maizels RM. White MPJ, et al. Immunology. 2020 Jul;160(3):248-260. doi: 10.1111/imm.13190. Epub 2020 Apr 19. Immunology. 2020. PMID: 32153025 Free PMC article. Review. - Regulation of allergy and autoimmunity in helminth infection.
Wilson MS, Maizels RM. Wilson MS, et al. Clin Rev Allergy Immunol. 2004 Feb;26(1):35-50. doi: 10.1385/CRIAI:26:1:35. Clin Rev Allergy Immunol. 2004. PMID: 14755074 Review.
Cited by
- Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection.
Giacomin PR, Siracusa MC, Walsh KP, Grencis RK, Kubo M, Comeau MR, Artis D. Giacomin PR, et al. J Immunol. 2012 Nov 1;189(9):4371-8. doi: 10.4049/jimmunol.1200691. Epub 2012 Sep 28. J Immunol. 2012. PMID: 23024277 Free PMC article. - Impact of Schistosoma japonicum infection on collagen-induced arthritis in DBA/1 mice: a murine model of human rheumatoid arthritis.
Song X, Shen J, Wen H, Zhong Z, Luo Q, Chu D, Qi Y, Xu Y, Wei W. Song X, et al. PLoS One. 2011;6(8):e23453. doi: 10.1371/journal.pone.0023453. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21858123 Free PMC article. - Modulation of goat monocyte function by HCcyst-2, a secreted cystatin from Haemonchus contortus.
Wang Y, Wen Y, Wang S, Ehsan M, Yan R, Song X, Xu L, Li X. Wang Y, et al. Oncotarget. 2017 Jul 4;8(27):44108-44120. doi: 10.18632/oncotarget.17308. Oncotarget. 2017. PMID: 28484087 Free PMC article. - Within-host mechanisms of immune regulation explain the contrasting dynamics of two helminth species in both single and dual infections.
Vanalli C, Mari L, Righetto L, Casagrandi R, Gatto M, Cattadori IM. Vanalli C, et al. PLoS Comput Biol. 2020 Nov 23;16(11):e1008438. doi: 10.1371/journal.pcbi.1008438. eCollection 2020 Nov. PLoS Comput Biol. 2020. PMID: 33226981 Free PMC article. - Schistosome infection promotes osteoclast-mediated bone loss.
Li W, Wei C, Xu L, Yu B, Chen Y, Lu D, Zhang L, Song X, Dong L, Zhou S, Xu Z, Zhu J, Chen X, Su C. Li W, et al. PLoS Pathog. 2021 Mar 18;17(3):e1009462. doi: 10.1371/journal.ppat.1009462. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33735306 Free PMC article.
References
- Bazzone L.E., Smith P.M., Rutitzky L.I., Shainheit M.G., Urban J.F., Setiawan T., Blum A.M., Weinstock J.V., Stadecker M.J. 2008. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis.Infect. Immun. 76:5164–5172 doi:<10.1128/IAI.00673-08> - DOI - PMC - PubMed
- Blount D., Hooi D., Feary J., Venn A., Telford G., Brown A., Britton J., Pritchard D. 2009. Immunological profiles of persons recruited for a randomized, placebo-controlled clinical trial of hookworm infection.Am. J. Trop. Med. Hyg. In press - PubMed