Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury - PubMed (original) (raw)
Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury
Marc R Suter et al. Mol Pain. 2009.
Abstract
Background: After peripheral nerve injury, spontaneous ectopic activity arising from the peripheral axons plays an important role in inducing central sensitization and neuropathic pain. Recent evidence indicates that activation of spinal cord microglia also contributes to the development of neuropathic pain. In particular, activation of p38 mitogen-activated protein kinase (MAPK) in spinal microglia is required for the development of mechanical allodynia. However, activity-dependent activation of microglia after nerve injury has not been fully addressed. To determine whether spontaneous activity from C- or A-fibers is required for microglial activation, we used resiniferatoxin (RTX) to block the conduction of transient receptor potential vanilloid subtype 1 (TRPV1) positive fibers (mostly C- and Adelta-fibers) and bupivacaine microspheres to block all fibers of the sciatic nerve in rats before spared nerve injury (SNI), and observed spinal microglial changes 2 days later.
Results: SNI induced robust mechanical allodynia and p38 activation in spinal microglia. SNI also induced marked cell proliferation in the spinal cord, and all the proliferating cells (BrdU+) were microglia (Iba1+). Bupivacaine induced a complete sensory and motor blockade and also significantly inhibited p38 activation and microglial proliferation in the spinal cord. In contrast, and although it produced an efficient nociceptive block, RTX failed to inhibit p38 activation and microglial proliferation in the spinal cord.
Conclusion: (1) Blocking peripheral input in TRPV1-positive fibers (presumably C-fibers) is not enough to prevent nerve injury-induced spinal microglial activation. (2) Peripheral input from large myelinated fibers is important for microglial activation. (3) Microglial activation is associated with mechanical allodynia.
Figures
Figure 1
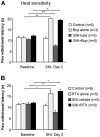
Effects of nerve block with bupivacaine microspheres (Bup, A) and resiniferatoxin (RTX, B) on heat sensitivity before and two days after spared nerve injury (SNI). Both bupivacaine and RTX increase the paw withdrawal latencies in injured and non injured rats. Note a decrease in latency after SNI. Baseline pain sensitivity was tested before drug treatment and nerve injury *p < 0.05, **p < 0.01.
Figure 2
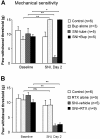
Effects of nerve block with bupivacaine microspheres (Bup, A) and resiniferatoxin (RTX, B) on mechanical sensitivity before and two days after spared nerve injury (SNI). SNI-induced mechanical allodynia, i.e. decrease in paw withdrawal threshold, is not prevented by RTX treatment. Baseline pain sensitivity was tested before drug treatment and nerve injury *p < 0.05, **p < 0.01.
Figure 3
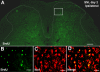
SNI induces microglial proliferation in the spinal cord. (A) Bromodeoxyuridine (BrdU) staining in the dorsal horn of the spinal cord two days after SNI. There is a dramatic increase in number of BrdU-positive profiles in the dorsal horn ipsilateral to nerve injury. Scale bar, 200 μm. (B-D) Enlargement of the ipsilateral medial dorsal horn (square indicated in A) showing co-localization of BrdU with the microglial marker Iba1. Scale bars, 50 μm.
Figure 4
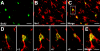
Confocal microscopy images show BrdU expression in spinal microglia two days after SNI. (A-C) Colocalization of BrdU and Iba1 in the medial superficial spinal cord. Scale bars, 50 μm. (D) Stack of confocal images (2 μm apart) from a double-labeled cell enlarged from a square in C. (E) Merge of all images in D. Scale bars, 20 μm.
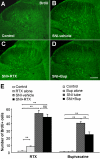
Figure 5
Bupivacaine but not resiniferatoxin reduces cell proliferation in the spinal cord dorsal horn after SNI. (A-D) BrdU immunostaining in the dorsal horn of control rats (A) and SNI rats receiving vehicle (B), RTX (C), and bupivacaine (Bup, D). (E) Number of BrdU-positive cell profiles in the dorsal horn: Left, effects of RTX on SNI-induced cell proliferation. Right: effects of bupivacaine on SNI-induced cell proliferation *p < 0.05, **p < 0.01, ns = no significance. Scale bar 200 μm. n = 3-5.
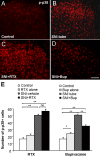
Figure 6
Bupivacaine but not resiniferatoxin reduces the phosphorylation of p38 MAPK in the spinal cord dorsal horn after SNI. (A-D) p-p38 immunostaining in the dorsal horn of control rats (A) and SNI rats receiving vehicle (B), RTX (C), and bupivacaine (Bup, D). (E) Number of p-p38-positive cell profiles in the dorsal horn: Left, effects of RTX on SNI-induced cell proliferation. Right: effects of bupivacaine on SNI-induced cell proliferation *p < 0.05, **p < 0.01, ns = no significance. Scale bar 100 μm. n = 3-5.
Similar articles
- Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model.
Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji RR. Wen YR, et al. Anesthesiology. 2007 Aug;107(2):312-21. doi: 10.1097/01.anes.0000270759.11086.e7. Anesthesiology. 2007. PMID: 17667577 - Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK.
Lee KM, Jeon SM, Cho HJ. Lee KM, et al. Eur J Pain. 2010 Aug;14(7):682.e1-12. doi: 10.1016/j.ejpain.2009.10.017. Epub 2009 Dec 2. Eur J Pain. 2010. PMID: 19959384 - p38 MAPK, microglial signaling, and neuropathic pain.
Ji RR, Suter MR. Ji RR, et al. Mol Pain. 2007 Nov 1;3:33. doi: 10.1186/1744-8069-3-33. Mol Pain. 2007. PMID: 17974036 Free PMC article. Review. - Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity.
Kissin I. Kissin I. Anesth Analg. 2008 Jul;107(1):271-81. doi: 10.1213/ane.0b013e318162cfa3. Anesth Analg. 2008. PMID: 18635498 Free PMC article. Review.
Cited by
- Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels.
Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED. Wilkerson JL, et al. Pain. 2012 May;153(5):1091-1106. doi: 10.1016/j.pain.2012.02.015. Epub 2012 Mar 17. Pain. 2012. PMID: 22425445 Free PMC article. - Microglial deactivation by adeno-associated virus expressing small-hairpin GCH1 has protective effects against neuropathic pain development in a spinothalamic tract-lesion model.
Jung HH, Koh CS, Park M, Kim JH, Woo HN, Lee H, Chang JW. Jung HH, et al. CNS Neurosci Ther. 2022 Jan;28(1):36-45. doi: 10.1111/cns.13751. Epub 2021 Nov 29. CNS Neurosci Ther. 2022. PMID: 34845843 Free PMC article. - Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers.
Shibata Y, Matsumoto Y, Kohno K, Nakashima Y, Tsuda M. Shibata Y, et al. Cells. 2025 May 2;14(9):666. doi: 10.3390/cells14090666. Cells. 2025. PMID: 40358190 Free PMC article. - Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia.
Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Kuhn MN, Zvonok AM, Thakur GA, Makriyannis A, Milligan ED. Wilkerson JL, et al. Brain Behav. 2012 Mar;2(2):155-77. doi: 10.1002/brb3.44. Brain Behav. 2012. PMID: 22574283 Free PMC article. - Macrophages and microglia in inflammation and neuroinflammation underlying different pain states.
Chen O, Luo X, Ji RR. Chen O, et al. Med Rev (2021). 2023 Nov 1;3(5):381-407. doi: 10.1515/mr-2023-0034. eCollection 2023 Oct. Med Rev (2021). 2023. PMID: 38283253 Free PMC article.
References
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editor. Textbook of Pain. Edinburgh: Churchill Livingstone; 1999. pp. 129–164.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054932/NS/NINDS NIH HHS/United States
- R01 NS067686/NS/NINDS NIH HHS/United States
- R03 TW007180/TW/FIC NIH HHS/United States
- DE17794/DE/NIDCR NIH HHS/United States
- NS67686/NS/NINDS NIH HHS/United States
- TW7180/TW/FIC NIH HHS/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- NS54932/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources