Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats - PubMed (original) (raw)
Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats
Miranda Brenneman et al. J Cereb Blood Flow Metab. 2010 Jan.
Abstract
We investigated intra-arterially administered autologous bone marrow mononuclear cells (MNCs) in rats with acute ischemic stroke. Long Evans rats (2 to 3 months or 12 months old) underwent tandem reversible common carotid artery (CCA)/middle cerebral artery (MCA) occlusion (CCAo/MCAo) for 3 h and then 24 h later underwent tibial bone marrow harvest. Ten million or 4 million cells were re-injected by an intra-carotid infusion. Control animals underwent marrow needle insertion and then saline injection into the carotid artery. Animals were assessed on a battery of neurological tests. MNCs in the ischemic brain were tracked using Q-dot nanocrystal labeling. Infarct volume and cytokines in the ischemia-affected brain were analyzed. Cell-treated animals in the younger and older groups showed improvement from 7 to 30 days after stroke compared with vehicle-treated animals. MNCs significantly reduced infarct volume compared with saline. There was a significant reduction in tumor necrosis factor-alpha, interleukin-1alpha (IL-1alpha), IL-beta, IL-6, and a significant increase in IL-10 in injured brains harvested from the cell-treated groups compared with saline controls. Labeled MNCs were found in the peri-infarcted area at 1 h and exponentially decreased over the ensuing week after injection. Autologous bone marrow MNCs can be safely harvested from rodents after stroke, migrate to the peri-infarct area, enhance recovery, and modulate the post-ischemic inflammatory response.
Figures
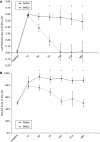
Figure 1
Young Long Evans rats treated with MNCs have improved recovery after cortical stroke. Animals underwent CCAo/MCAo, and 24 h later either received an IA injection of autologous MNCs or saline followed by neurological testing for 30 days. (A) The cylinder test demonstrates preferential left forearm placement in animals that have undergone left CCAo/MCAo and IA saline injection at 24 h after stroke. Deficits persist up to 28 days after stroke. Animals that receive MNCs, however, at 24 h after stroke show resolution of deficits over time (P<0.05 for days 7, 14, 21, and 28; _n_=7 per group). (B) The corner test demonstrates preferential turning to the left in animals that have undergone left CCAo/MCAo and IA saline injection at 24 h after stroke. Deficits persist up to 28 days after stroke. Animals that receive MNCs, however, at 24 h after stroke show resolution of deficits over time (P<0.05 for days 14, 21, and 28; _n_=7 per group).
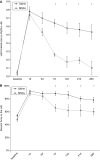
Figure 2
Twelve-month-old, Long Evans rats treated with MNCs show improved recovery after cortical stroke. Retired breeders underwent CCAo/MCAo, and 24 h later either received an IA injection of autologous MNCs or saline. Saline-treated rats show persistent severe deficits lasting for at least 28 days as determined using cylinder (A) and corner (B) tests. Cell-treated animals show significant reductions in neurological deficits on the cylinder (A) and corner (B) tests starting at 7 days after stroke (_n_=5 per group).
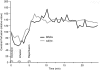
Figure 3
Cerebral perfusion during IA infusion of MNCs or saline. At 24 h after acute stroke, the external carotid and common artery were ligated, which corresponded with a decline in cerebral perfusion as usual in this model (labeled occlusion). Then a catheter was inserted to the ICA to infuse saline or MNCs in a final volume of 1 ml (labeled infusion). In response to the infusion of MNCs or saline, the cerebral perfusion increased in all animals tested beyond baseline. The CCA was unclamped (labeled reperfusion) and then there was a return of cerebral perfusion to mildly elevated levels in both saline- and MNC-treated groups. Data represent the mean of four animals per group.
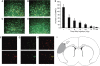
Figure 4
Twenty-four hours after CCAo/MCAo, Long Evans rats received an intra-carotid injection of MNCs. The MNCs were derived from the bone marrow and labeled with Q tracker nanocrystals before infusion. Fluorescence microscopic images illustrate time-dependent elimination of MNCs in per-infarcted rat brain. One hour (A), 6 h (B), 24 h (C), and 7 days (D) after intra-carotid injection. Green: Fluorescein isothiocyanate (to unspecifically label the brain) and red: Q-Tracker. Magnification, × 40. (E) A histogram showing a reduction in the number of labeled MNCs in the peri-infarcted zone as a function of time (_n_=4 per group). (F to H) Fluorescence microscopic images of two different areas in the peri-infarct region illustrating Q-dot-labeled MNCs (left panel), TUNEL-stained cells in the same region (middle panel), and colocalization of Q-MNCs with TUNEL-positive immunoreactivity. Colocalization of Q-MNCs with TUNEL is represented by yellow fluorescence due to merging of red fluorescence with the green fluorescence (right panel). Magnification, × 40. (I) A diagram to show the borderzone area of the infarct (peri-infarct) where samples were obtained for histological analysis.
Figure 5
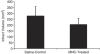
A bar graph exhibiting significantly (P<0.03) reduced infarct volume in MNC-treated rats as compared with saline-treated control animals. Data are mean±s.d. (_n_=5 per group).
Figure 6
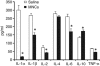
Ischemic brain tissue after stroke treated with MNCs or saline was homogenized, centrifuged, and then the supernatant was analyzed using a multiplex bead-based enzyme-linked immunosorbant assay for the presence of selective cytokines. As shown, levels of several proinflammatory cytokines were significantly lower (_n_=5, P<0.05) in tissue samples taken from cell-treated animals as compared with that in saline-treated controls, whereas that of the anti-inflammatory cytokine, IL-10, was significantly increased (_n_=5, P<0.05).
Similar articles
- Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke.
Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, Grotta JC, Aronowski J, Savitz SI. Yang B, et al. J Neurosci Res. 2011 Jun;89(6):833-9. doi: 10.1002/jnr.22614. Epub 2011 Mar 15. J Neurosci Res. 2011. PMID: 21412816 Free PMC article. - Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke.
Yang B, Migliati E, Parsha K, Schaar K, Xi X, Aronowski J, Savitz SI. Yang B, et al. Stroke. 2013 Dec;44(12):3463-72. doi: 10.1161/STROKEAHA.111.000821. Epub 2013 Oct 10. Stroke. 2013. PMID: 24114454 Free PMC article. - Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial.
Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-León M, de la Torre J, Gamero MA, Tamayo JA, Ochoa-Sepúlveda JJ, Maestre J, Carmona M, Piñero P, Calderón-Cabrera C, Jimenez MD, Gonzalez A, Montaner J. Moniche F, et al. Int J Stroke. 2015 Oct;10(7):1149-52. doi: 10.1111/ijs.12520. Epub 2015 Jun 4. Int J Stroke. 2015. PMID: 26044701 Clinical Trial. - Ischemic stroke may activate bone marrow mononuclear cells to enhance recovery after stroke.
Yang B, Xi X, Aronowski J, Savitz SI. Yang B, et al. Stem Cells Dev. 2012 Dec 10;21(18):3332-40. doi: 10.1089/scd.2012.0037. Epub 2012 Aug 3. Stem Cells Dev. 2012. PMID: 22731389 Free PMC article. - Valproic acid enhances the effect of bone marrow-derived mononuclear cells in a rat ischemic stroke model.
Suda S, Katsura KI, Saito M, Kamiya N, Katayama Y. Suda S, et al. Brain Res. 2014 May 27;1565:74-81. doi: 10.1016/j.brainres.2014.04.011. Epub 2014 Apr 16. Brain Res. 2014. PMID: 24746498
Cited by
- CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice.
Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J. Wang J, et al. Brain Behav Immun. 2015 Mar;45:98-108. doi: 10.1016/j.bbi.2014.12.015. Epub 2014 Dec 16. Brain Behav Immun. 2015. PMID: 25526817 Free PMC article. - A view of the genetic and proteomic profile of extracellular matrix molecules in aging and stroke.
Chmelova M, Androvic P, Kirdajova D, Tureckova J, Kriska J, Valihrach L, Anderova M, Vargova L. Chmelova M, et al. Front Cell Neurosci. 2023 Nov 30;17:1296455. doi: 10.3389/fncel.2023.1296455. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38107409 Free PMC article. - Intracarotid administration of human bone marrow mononuclear cells in rat photothrombotic ischemia.
Minnerup J, Seeger FH, Kuhnert K, Diederich K, Schilling M, Dimmeler S, Schäbitz WR. Minnerup J, et al. Exp Transl Stroke Med. 2010 Feb 2;2(1):3. doi: 10.1186/2040-7378-2-3. Exp Transl Stroke Med. 2010. PMID: 20298535 Free PMC article. - Intra-Arterial Delivery of Cell Therapies for Stroke.
Guzman R, Janowski M, Walczak P. Guzman R, et al. Stroke. 2018 May;49(5):1075-1082. doi: 10.1161/STROKEAHA.117.018288. Epub 2018 Apr 18. Stroke. 2018. PMID: 29669876 Free PMC article. Review. No abstract available. - Influence of Bone Marrow-Derived Mesenchymal Stem Cell Therapy on Oxidative Stress Intensity in Minimally Conscious State Patients.
Jezierska-Wozniak K, Sinderewicz E, Czelejewska W, Wojtacha P, Barczewska M, Maksymowicz W. Jezierska-Wozniak K, et al. J Clin Med. 2020 Mar 3;9(3):683. doi: 10.3390/jcm9030683. J Clin Med. 2020. PMID: 32138308 Free PMC article.
References
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. - PubMed
- Baker AH, Sica V, Work LM, Williams-Ignarro S, de Nigris F, Lerman LO, Casamassimi A, Lanza A, Schiano C, Rienzo M, Ignarro LJ, Napoli C. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci USA. 2007;104:3597–3602. - PMC - PubMed
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. - PubMed
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical