Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease - PubMed (original) (raw)
Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease
Caterina P Minniti et al. Br J Haematol. 2009 Dec.
Abstract
Pulmonary Hypertension is a serious complication of sickle cell disease (SCD), with high morbidity and mortality. Endothelin (ET)-1, a potent vasoconstrictor elevated in SCD, acts through the ET receptors (ETR), ETR-A and ETR-B. Bosentan and ambrisentan are ETR blockers used in primary pulmonary hypertension. We report on the use of ETR blocking agents in a cohort of 14 high-risk SCD adult patients with pulmonary hypertension. Patients underwent right heart catheterization, 6-min walk test, echocardiogram, physical examination and blood work-up before starting ETR blockers. Eight patients received ETR blockers as initial therapy; six patients were already taking sildenafil. Over more than 6 months of therapy, sequential measurements of 6-min walk distance increased significantly (baseline 357 +/- 22 to 398 +/- 18 m at 5-6 months, P < 0.05). Downward trends were observed for amino-terminal brain natriuretic peptide and tricuspid regurgitant velocity. Pulmonary artery mean pressures decreased in three patients that had repeat right heart catheterization (44-38 mmHg). Adverse events were: increased serum alanine aminotransferase (2), peripheral oedema (4), rash (1), headache (3), decreased haemoglobin (2). Therapy was stopped in two patients who were switched then to the other ETR blocker agent. These data suggest preliminary evidence for the benefit of bosentan and ambrisentan in pulmonary hypertension in SCD.
Figures
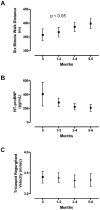
Figure 1
Changes in 6-min walk distance (A), NT-proBNP a serum levels (B) and tricuspid regurgitant velocity as measured by echocardiogram (C) during the time of observation on ETR blocking therapy (6–14 months, mean 8.5 months). Sequential measurements of 6-min walk distance increased significantly (mean ± standard error baseline, 357 ± 22 m; 1–2 months, 367 ± 17 m; 3–4 months, 387 ± 16 m; 5–6 months, 398 ± 18 m; n = 12, P < 0.05, ANOVA with repeated measures).
Similar articles
- [Endothelin receptor antagonists in the new European guidelines on pulmonary hypertension].
Montani D, Humbert M. Montani D, et al. Rev Mal Respir. 2010 Feb;27(2):103-5. doi: 10.1016/j.rmr.2009.12.007. Epub 2010 Jan 25. Rev Mal Respir. 2010. PMID: 20206056 French. No abstract available. - Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension.
Mathai SC, Girgis RE, Fisher MR, Champion HC, Housten-Harris T, Zaiman A, Hassoun PM. Mathai SC, et al. Eur Respir J. 2007 Mar;29(3):469-75. doi: 10.1183/09031936.00081706. Epub 2006 Nov 1. Eur Respir J. 2007. PMID: 17079256 - Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities.
McGoon MD, Frost AE, Oudiz RJ, Badesch DB, Galie N, Olschewski H, McLaughlin VV, Gerber MJ, Dufton C, Despain DJ, Rubin LJ. McGoon MD, et al. Chest. 2009 Jan;135(1):122-129. doi: 10.1378/chest.08-1028. Epub 2008 Sep 23. Chest. 2009. PMID: 18812445 Clinical Trial. - Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension.
Aversa M, Porter S, Granton J. Aversa M, et al. Drug Saf. 2015 May;38(5):419-35. doi: 10.1007/s40264-015-0275-y. Drug Saf. 2015. PMID: 25792028 Review. - Endothelin receptor antagonism: role in the treatment of pulmonary arterial hypertension related to scleroderma.
Kabunga P, Coghlan G. Kabunga P, et al. Drugs. 2008;68(12):1635-45. doi: 10.2165/00003495-200868120-00003. Drugs. 2008. PMID: 18681488 Review.
Cited by
- The Cardiopulmonary Complications of Sickle Cell Disease.
Desai AA, Machado RF, Cohen RT. Desai AA, et al. Hematol Oncol Clin North Am. 2022 Dec;36(6):1217-1237. doi: 10.1016/j.hoc.2022.07.014. Hematol Oncol Clin North Am. 2022. PMID: 36400540 Free PMC article. Review. - Saudi Guidelines on the Diagnosis and Treatment of Pulmonary Hypertension: Pulmonary hypertension associated with hemolytic anemia.
Saleemi S. Saleemi S. Ann Thorac Med. 2014 Jul;9(Suppl 1):S67-73. doi: 10.4103/1817-1737.134039. Ann Thorac Med. 2014. PMID: 25077000 Free PMC article. - Pulmonary complications of sickle cell disease.
Miller AC, Gladwin MT. Miller AC, et al. Am J Respir Crit Care Med. 2012 Jun 1;185(11):1154-65. doi: 10.1164/rccm.201111-2082CI. Epub 2012 Mar 23. Am J Respir Crit Care Med. 2012. PMID: 22447965 Free PMC article. Review. - Focused Update on Pulmonary Hypertension in Children-Selected Topics of Interest for the Adult Cardiologist.
Albinni S, Marx M, Lang IM. Albinni S, et al. Medicina (Kaunas). 2020 Aug 19;56(9):420. doi: 10.3390/medicina56090420. Medicina (Kaunas). 2020. PMID: 32825190 Free PMC article. Review. - American Society of Hematology 2019 guidelines for sickle cell disease: cardiopulmonary and kidney disease.
Liem RI, Lanzkron S, D Coates T, DeCastro L, Desai AA, Ataga KI, Cohen RT, Haynes J, Osunkwo I, Lebensburger JD, Lash JP, Wun T, Verhovsek M, Ontala E, Blaylark R, Alahdab F, Katabi A, Mustafa RA. Liem RI, et al. Blood Adv. 2019 Dec 10;3(23):3867-3897. doi: 10.1182/bloodadvances.2019000916. Blood Adv. 2019. PMID: 31794601 Free PMC article.
References
- Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC, Stocker JW. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. - PubMed
- Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. - PubMed
- Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61:227–237. - PubMed
- Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ZIA HL005074-05/ImNIH/Intramural NIH HHS/United States
- ZIA HL005074-04/ImNIH/Intramural NIH HHS/United States
- ZIA HL006014/ImNIH/Intramural NIH HHS/United States
- ZIA HL005074/ImNIH/Intramural NIH HHS/United States
- ZIA HL006014-01/ImNIH/Intramural NIH HHS/United States
- ZIA HL006014-02/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical