Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission - PubMed (original) (raw)
Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission
Rajesh Ramachandran et al. Mol Biol Cell. 2009 Nov.
Abstract
The GTPase dynamin catalyzes the scission of deeply invaginated clathrin-coated pits at the plasma membrane, but the mechanisms governing dynamin-mediated membrane fission remain poorly understood. Through mutagenesis, we have altered the hydrophobic nature of the membrane-inserting variable loop 1 (VL1) of the pleckstrin homology (PH) domain of dynamin-1 and demonstrate that its stable insertion into the lipid bilayer is critical for high membrane curvature generation and subsequent membrane fission. Dynamin PH domain mutants defective in curvature generation regain function when assayed on precurved membrane templates in vitro, but they remain defective in the scission of clathrin-coated pits in vivo. These results demonstrate that, in concert with dynamin self-assembly, PH domain membrane insertion is essential for fission and vesicle release in vitro and for clathrin-mediated endocytosis in vivo.
Figures
Figure 1.
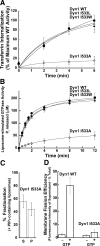
I533C mutation in VL1 impairs dynamin function in vivo and in vitro. (A) Transferrin uptake was followed in tTA-HeLa cells infected for 16–18 h with recombinant adenovirus encoding Dyn1 WT (●), RCLDyn1 (○), or RCLDyn1 I533C (■). The kinetics of internalization is plotted as a percentage of maximum uptake by Dyn1 WT. (B) Lipid-stimulated GTPase activities for 0.5 μM Dyn1 WT (●), RCLDyn1 (○), RCLDyn1 I533C (■), or RCLDyn1 I533C-NBD (□) preincubated with PIP2-containing liposomes (150 μM total lipid) were measured at 37°C. The concentration of Pi released is plotted as a function of time. (C) Binding of 1.0 μM Dyn1 WT, RCLDyn1 I533C, or RCLDyn1 I533C-NBD to PIP2-containing liposomes (300 μM lipid) was examined by sedimentation followed by SDS-PAGE analyses of supernatant (S) and pellet (P) fractions. Densitometric analyses of S and P fractions obtained using an Alpha Innotec Imager and FluorChem SP software (San Leandro, CA) are plotted. (D) Membrane fission activities of 0.5 μM Dyn 1 WT, unlabeled RCLDyn1 I533C, or fluorescently labeled RCLDyn1 I533C-NBD on RhPE-labeled SUPER templates (6 μM total lipid) in the constant presence of GTP (1 mM) were determined by a low-speed sedimentation assay as described in Materials and Methods. The fluorescence intensity of RhPE-labeled vesicles generated by membrane fission and recovered in the supernatant is plotted as a percentage of the total RhPE-labeled templates added to the assay. All values reported in this study represent the mean ± SD of at least three independent experiments.
Figure 2.
Hydrophobic VL1-membrane interactions are critical for dynamin function. (A) Kinetics of transferrin uptake in cells expressing Dyn1 WT (●), Dyn1 I533W (▴), Dyn1 I533L (▵), or Dyn1 I533A (◊) were determined and plotted as in Figure 1A. (B) Lipid-stimulated GTPase activities of 0.5 μM Dyn1 WT (●), Dyn1 I533W (▴), Dyn1 I533L (▵), or Dyn1 I533A (◊) preincubated with PIP2-containing liposomes (150 μM total lipid) were obtained and plotted as in Figure 1B. (C) Sedimentation analysis of 1.0 μM Dyn1 I533A on PIP2-containing liposomes (150 μM total lipid) was performed and plotted as in Figure 1C. (D) Membrane fission activities of 0.5 μM Dyn1 WT and Dyn1 I533A was determined on SUPER templates (6 μM total lipid) in the constant presence of GTP (1 mM) by a low-speed sedimentation assay as in Figure 1D.
Figure 3.
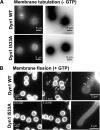
I533A VL1 mutant is defective in membrane curvature generation and membrane fission. (A) Fluorescence images of RhPE-labeled SUPER templates showing the effect of addition of either 0.5 μM Dyn1 WT or Dyn1 I533A in the absence of GTP. Images were acquired at a focal plane close to the coverslip. Contrast inverted images are also shown (right panels) for clarity. Note the absence of optically visible membrane tubules for Dyn1 I533A. (B) Fluorescence images showing the effect of addition of either 0.5 μM Dyn1 WT or Dyn1 I533A in the constant presence of GTP to membrane tubules (typically 0.5–1.0 μm in diameter) transiently generated by glycerol flow from RhPE-labeled SUPER templates (Pucadyil and Schmid, 2008). Left and middle, note the time-dependent release of vesicles (arrows) from the templates only in the case of Dyn1 WT. See Supplemental Movie 1 for entire sequence. Right, magnified fluorescence images showing visible membrane constrictions (arrows) before fission of flow-generated membrane tubules only in the case of Dyn1 WT.
Figure 4.
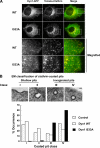
Dyn1 I533A targets to but fails to mediate fission of CCPs. (A) Top panels, live cell epifluorescence imaging of retrovirus-infected Dyn2 KO mouse fibroblasts expressing Tomato-clathrin light chain along with GFP fusion constructs of either Dyn1 WT or Dyn1 I533A. Scale bar, 10 μm. Bottom panels, magnified images from insets on top panels that demonstrate colocalization of clathrin and dynamin. (B) Top, EM classification of CCP profiles according to degree of membrane curvature and invagination. Classes I and II represent shallow, whereas classes III and IV represent deeply invaginated coated pits that remain tethered to the plasma membrane via a narrow membrane neck. Scale bar, 100 nm. Bottom, percent occurrence of these classes in tTA-HeLa cells overexpressing either Dyn1 WT (□) or Dyn1 I533A (■) or neither (▧) is plotted. Greater than 100 CCP profiles were scored for each sample.
Figure 5.
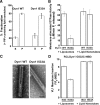
Precurved lipid templates rescue Dyn1 I533A. (A) Sedimentation analysis of 1.0 μM Dyn1 I533A on preformed PIP2-containing lipid nanotubes (300 μM total lipid) was performed and plotted as in Figure 1C. (B) Assembly-stimulated GTP hydrolysis rate of 0.5 μM Dyn1 WT or Dyn1 I533A induced upon preincubation with either PIP2-containing liposomes or PIP2-containing lipid nanotubes (150 μM total lipid) is plotted as μM Pi released per minute. (C) Representative electron micrographs of Dyn1 WT and Dyn1 I533A self-assembled on PIP2-containing lipid nanotubes. Scale bar, 50 nm. (D) The magnitude of VL1-membrane binding in 0.1 μM RCLDyn1 (G532C-NBD), I533 or I533A, was determined by detecting the fold increase in the steady-state emission intensity of NBD upon incubation with either PIP2-containing liposomes or PIP2-containing lipid nanotubes (30 μM total lipid). F/F0 is the ratio of the emission intensity values obtained for NBD before (F0) and after (F) incubation with the corresponding lipid templates.
Figure 6.
Fission of precurved membrane tubes mediated by Dyn1 I533A. Time-lapse fluorescence images showing the effect of addition of either 0.5 μM Dyn1 WT or Dyn1 I533A to membrane tethers (typically ∼50–100-nm diameter) pulled out of RhPE-labeled SUPER templates in the presence of GTP (1 mM final). See Supplemental Movie 2 for entire sequence.
Comment in
- Mol Biol Cell. 20:4629.
Similar articles
- Building a fission machine--structural insights into dynamin assembly and activation.
Chappie JS, Dyda F. Chappie JS, et al. J Cell Sci. 2013 Jul 1;126(Pt 13):2773-84. doi: 10.1242/jcs.108845. Epub 2013 Jun 18. J Cell Sci. 2013. PMID: 23781021 Free PMC article. Review. - Alternate pleckstrin homology domain orientations regulate dynamin-catalyzed membrane fission.
Mehrotra N, Nichols J, Ramachandran R. Mehrotra N, et al. Mol Biol Cell. 2014 Mar;25(6):879-90. doi: 10.1091/mbc.E13-09-0548. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478459 Free PMC article. - The role of dynamin and its binding partners in coated pit invagination and scission.
Hill E, van Der Kaay J, Downes CP, Smythe E. Hill E, et al. J Cell Biol. 2001 Jan 22;152(2):309-23. doi: 10.1083/jcb.152.2.309. J Cell Biol. 2001. PMID: 11266448 Free PMC article. - SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis.
Soulet F, Yarar D, Leonard M, Schmid SL. Soulet F, et al. Mol Biol Cell. 2005 Apr;16(4):2058-67. doi: 10.1091/mbc.e04-11-1016. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703209 Free PMC article. - Endocytosis: clathrin-mediated membrane budding.
Ungewickell EJ, Hinrichsen L. Ungewickell EJ, et al. Curr Opin Cell Biol. 2007 Aug;19(4):417-25. doi: 10.1016/j.ceb.2007.05.003. Epub 2007 Jul 13. Curr Opin Cell Biol. 2007. PMID: 17631994 Review.
Cited by
- Building a fission machine--structural insights into dynamin assembly and activation.
Chappie JS, Dyda F. Chappie JS, et al. J Cell Sci. 2013 Jul 1;126(Pt 13):2773-84. doi: 10.1242/jcs.108845. Epub 2013 Jun 18. J Cell Sci. 2013. PMID: 23781021 Free PMC article. Review. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Constricting membranes at the nano and micro scale.
Mooren OL, Schafer DA. Mooren OL, et al. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20559-60. doi: 10.1073/pnas.0911630106. Epub 2009 Dec 1. Proc Natl Acad Sci U S A. 2009. PMID: 19955440 Free PMC article. No abstract available. - Mutation of key lysine residues in the Insert B region of the yeast dynamin Vps1 disrupts lipid binding and causes defects in endocytosis.
Smaczynska-de Rooij II, Marklew CJ, Palmer SE, Allwood EG, Ayscough KR. Smaczynska-de Rooij II, et al. PLoS One. 2019 Apr 22;14(4):e0215102. doi: 10.1371/journal.pone.0215102. eCollection 2019. PLoS One. 2019. PMID: 31009484 Free PMC article. - Biological Cargo: Exosomes and their Role in Cancer Progression and Metastasis.
Tripathi S, Sharma Y, Kumar D. Tripathi S, et al. Curr Top Med Chem. 2025;25(3):263-285. doi: 10.2174/0115680266304636240626055711. Curr Top Med Chem. 2025. PMID: 38984577 Review.
References
- Antonny B. Membrane deformation by protein coats. Curr. Opin. Cell Biol. 2006;18:386–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources