Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment - PubMed (original) (raw)
Review
Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment
Rukhsana Sultana et al. J Bioenerg Biomembr. 2009 Oct.
Abstract
Alzheimer disease (AD) is an age-related neurodegenerative disorder, characterized histopathologically by the presence of senile plaques (SP), neurofibrillary tangles and synapse loss in selected brain regions. Positron emission tomography (PET) studies of glucose metabolism revealed decreased energetics in brain of subjects with AD and arguably its earliest form, mild cognitive impairment (MCI), and this decrease correlated with brain structural studies using MRI. The main component of senile plaques is amyloid beta-peptide (Abeta), a 40-42 amino acid peptide that as oligomers is capable of inducing oxidative stress under both in vitro and in vivo conditions and is neurotoxic. In the mitochondria isolated from AD brain, Abeta oligomers that correlated with the reported increased oxidative stress markers in AD have been reported. The markers of oxidative stress have been localized in the brain regions of AD and MCI that show pathological hallmarks of this disease, suggesting the possible role of Abeta in the initiation of the free-radical mediated process and consequently to the build up oxidative stress and AD pathogenesis. Using redox proteomics our laboratory found a number of oxidatively modified brain proteins that are directly in or are associated with the mitochondrial proteome, consistent with a possible involvement of the mitochondrial targeted oxidatively modified proteins in AD progression or pathogenesis. The precise mechanistic link between mitochondrial oxidative damage and role of oligomeric Abeta has not been explicated. In this review, we discuss the role of the oxidation of mitochondria-relevant brain proteins to the pathogenesis and progression of AD.
Figures
Fig. 1
Oxidatively modified mitochondria-relevant proteins in AD and MCI brain
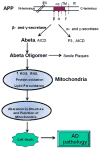
Fig. 2
Consequence of Aβ(1-42) on mitochondria and AD pathogenesis. See text. EC extra cellular, IC intracellualr, TM transmembrane, ROS reactive oxygen species, RNS reactive nitrogen species
Similar articles
- Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis.
Sultana R, Perluigi M, Butterfield DA. Sultana R, et al. Acta Neuropathol. 2009 Jul;118(1):131-50. doi: 10.1007/s00401-009-0517-0. Epub 2009 Mar 14. Acta Neuropathol. 2009. PMID: 19288120 Free PMC article. Review. - Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment.
Butterfield DA, Reed T, Newman SF, Sultana R. Butterfield DA, et al. Free Radic Biol Med. 2007 Sep 1;43(5):658-77. doi: 10.1016/j.freeradbiomed.2007.05.037. Epub 2007 Jun 13. Free Radic Biol Med. 2007. PMID: 17664130 Free PMC article. Review. - Redox proteomics and amyloid β-peptide: insights into Alzheimer disease.
Butterfield DA, Boyd-Kimball D. Butterfield DA, et al. J Neurochem. 2019 Nov;151(4):459-487. doi: 10.1111/jnc.14589. Epub 2018 Nov 27. J Neurochem. 2019. PMID: 30216447 Free PMC article. Review. - Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression.
Butterfield DA, Swomley AM, Sultana R. Butterfield DA, et al. Antioxid Redox Signal. 2013 Sep 10;19(8):823-35. doi: 10.1089/ars.2012.5027. Epub 2013 Feb 14. Antioxid Redox Signal. 2013. PMID: 23249141 Free PMC article. Review.
Cited by
- Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain.
Sultana R, Perluigi M, Butterfield DA. Sultana R, et al. Free Radic Biol Med. 2013 Sep;62:157-169. doi: 10.1016/j.freeradbiomed.2012.09.027. Epub 2012 Oct 5. Free Radic Biol Med. 2013. PMID: 23044265 Free PMC article. Review. - Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease.
Sultana R, Baglioni M, Cecchetti R, Cai J, Klein JB, Bastiani P, Ruggiero C, Mecocci P, Butterfield DA. Sultana R, et al. Free Radic Biol Med. 2013 Dec;65:595-606. doi: 10.1016/j.freeradbiomed.2013.08.001. Epub 2013 Aug 8. Free Radic Biol Med. 2013. PMID: 23933528 Free PMC article. - Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (-)-epigallocatechin-3-gallate.
He Y, Cui J, Lee JC, Ding S, Chalimoniuk M, Simonyi A, Sun AY, Gu Z, Weisman GA, Wood WG, Sun GY. He Y, et al. ASN Neuro. 2011 Feb 8;3(1):e00050. doi: 10.1042/AN20100025. ASN Neuro. 2011. PMID: 21434871 Free PMC article. - Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: implications for low vitamin D-dependent age-related cognitive decline.
Keeney JTR, Förster S, Sultana R, Brewer LD, Latimer CS, Cai J, Klein JB, Porter NM, Butterfield DA. Keeney JTR, et al. Free Radic Biol Med. 2013 Dec;65:324-334. doi: 10.1016/j.freeradbiomed.2013.07.019. Epub 2013 Jul 18. Free Radic Biol Med. 2013. PMID: 23872023 Free PMC article. - Intraneuronal β-amyloid impaired mitochondrial proteostasis through the impact on LONP1.
Wang W, Ma X, Bhatta S, Shao C, Zhao F, Fujioka H, Torres S, Wu F, Zhu X. Wang W, et al. Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2316823120. doi: 10.1073/pnas.2316823120. Epub 2023 Dec 13. Proc Natl Acad Sci U S A. 2023. PMID: 38091289 Free PMC article.
References
- Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. J Neurochem. 2000;74:2520–2527. - PubMed
- Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. J Bioenerg Biomembr. 2005;37:207–225. - PubMed
- Atamna H, Frey WH., 2nd Mitochondrion. 2007;7:297–310. - PubMed
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Neurobiol Aging. 2002;23:371–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010836/AG/NIA NIH HHS/United States
- P01 AG010836-10/AG/NIA NIH HHS/United States
- AG-10836/AG/NIA NIH HHS/United States
- P01 AG005119-19/AG/NIA NIH HHS/United States
- P01 AG005119/AG/NIA NIH HHS/United States
- AG-05119/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical