DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212 - PubMed (original) (raw)
DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212
Ju Young Kim et al. Neuron. 2009.
Abstract
Disrupted-in-schizophrenia 1 (DISC1), a susceptibility gene for major mental illnesses, regulates multiple aspects of embryonic and adult neurogenesis. Here, we show that DISC1 suppression in newborn neurons of the adult hippocampus leads to overactivated signaling of AKT, another schizophrenia susceptibility gene. Mechanistically, DISC1 directly interacts with KIAA1212, an AKT binding partner that enhances AKT signaling in the absence of DISC1, and DISC1 binding to KIAA1212 prevents AKT activation in vitro. Functionally, multiple genetic manipulations to enhance AKT signaling in adult-born neurons in vivo exhibit similar defects as DISC1 suppression in neuronal development that can be rescued by pharmacological inhibition of mammalian target of rapamycin (mTOR), an AKT downstream effector. Our study identifies the AKT-mTOR signaling pathway as a critical DISC1 target in regulating neuronal development and provides a framework for understanding how multiple susceptibility genes may functionally converge onto a common pathway in contributing to the etiology of certain psychiatric disorders.
Figures
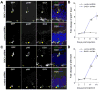
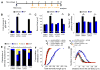
Figure 1. Over-activation of AKT signaling in newborn neurons with DISC1 knockdown in the adult brain
(A) Sample confocal images of immunostainining for GFP, phosphorylated AKT (pAKT), an immature neuronal marker DCX, and DAPI. Retroviruses co-expressing GFP and DISC1-shRNA, or a control-shRNA, were used to infect proliferating neural progenitors in the adult brain. GFP+ neurons were examined at 14 days post-viral injection. Scale bar: 25 μm. (B) A summary of quantifications of pAKT levels in the cytosol of GFP+DCX+ immature neurons at different stages of development in the adult brain. The pAKT fluorescence intensity of individual GFP+DCX+ new neuron was first normalized to the GFP-DCX+ immature new neurons in the same image. Values represent mean ± SEM (n = 10-33 cells from at least four animals; *: P < 0.01, ANOVA) (C and D) Same as in (A and B), except that phosphorylated S6 (pS6) was examined.
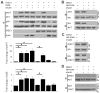
Figure 2. Direct interaction between DISC1 and KIAA1212
(A and B) Association of DISC1 and KIAA1212 in hippocampal neurons. Lysates from primary hippocampal neurons in culture (A) and acutely dissected hippocampal tissues (B) were subjected to co-IP using antibodies against DISC1 or KIAA1212 (KIAA), or using control antibodies, followed by Western Blot analysis. (C and D) Mapping of KIAA1212 domains involved in direct association with DISC1. Shown in (C) is a schematic diagram of different domains and regions of KIAA1212 used to generate GST-tagged recombinant fragments. Shown in (D) is a sample Western Blot of the in vitro pull-down of recombinant His-tagged DISC1 by different recombinant GST-KIAA1212 fragments. (E) Direct association between DISC1 and the CT2 domain of KIAA1212. A fixed amount of 6xHis-DISC1 was mixed with various amount of GST-CT2. Amounts of bound and free DISC1 were quantified. The percentage of bound DISC1 was plotted against the concentration of CT2 and Kd value was calculated by the Scatchard analysis.
Figure 3. Modulation of AKT signaling by DISC1 through KIAA1212
(A) Western Blot analysis of AKT and S6 signaling. HEK293 cells transfected with expression vectors for HA-KIAA1212, myc-DISC1, or co-transfected with both, were serum starved overnight and then treated with insulin (100 nM) or saline for 15 mins. Cell lysates were subjected to Western Blot analysis for phosphorylated and total endogenous AKT, phosphorylated and total endogenous S6, myc-DISC1 (DISC1) and HA-KIAA1212 (KIAA), respectively. Shown are sample blots and summaries of quantifications of the levels of AKT and S6 phosphorylation. For each condition, a value was first obtained for the ratio of phosphorylated over total amount of AKT or S6, which was then normalized to those without overexpression and insulin treatment for each individual experiment. Values represent mean ± SEM (n = 5 experiments; *: P < 0.01, ANOVA). (B) Inhibition of association between KIAA1212 and AKT by DISC1. HEK293 cells were transfected with expression constructs for HA-KIAA1212, or for both HA-KIAA1212 and myc-DISC1. Cultures were serum starved overnight and then treated with insulin (100 nM) or saline for 15 mins. Cell lysates were subjected to co-IP using antibodies against HA and followed by immunoblotting for AKT, or vice versa. (C) Association between KIAA1212 and DISC1. Similar as (A), HEK293 cells were co-transfected with expression constructs for HA-KIAA1212 and DISC1. Cell lysates were subjected to co-IP using antibodies against HA and followed by immunoblotting for DISC1, or vice versa. (D) Lack of strong association between DISC1 and AKT. Similar as in (A), HEK293 cells were transfected with expression constructs for DISC1, or for both DISC1 and KIAA1212. Cell lysates were subjected to co-IP using antibodies against AKT and followed by immunoblotting for DISC1, or vice versa.
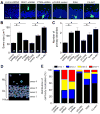
Figure 4. Role of KIAA1212 and AKT signaling in regulating morphogenesis and positioning of newborn neurons in the adult brain
(A to C) Morphogenesis of new neurons in the adult brain. Engineered retroviruses were used for birth-dating, lineage tracing with GFP expression, and genetic manipulation with expression of the transgene or shRNA. Two different control viruses, control-shRNA and pCUXIE (vector for transgene expression), were used for comparison of expression of shRNAs and transgenes, respectively. GFP+ neurons were examined at 14 dpi. Shown in (A) are sample single section confocal images of GFP+ and DAPI under different manipulations. Scale bar: 25 μm. Also shown are summaries of the soma size (B) and number of primary dendrites (C) of GFP+ neurons under different conditions. Numbers associated with each bar graph indicate the number of neurons examined from at least four animals under each condition. Values represent mean ± SEM (*: P < 0.01, ANOVA). (D and E) Positioning of newborn neurons in the dentate gyrus of the adult hippocampus. Shown in (D) is a schematic diagram of four areas defined for the dentate gyrus region. GL: granule cell layer; area 1: inner granule cell layer; area 2: middle granule cell layer; area 3: outer granule cell layer; ML: molecular layer (area 4). Shown in (E) is a summary of the percentile distribution of GFP+ neuron cell body within each domain as defined in (D). The same groups of GFP+ neurons as in (B) and (C) were used for quantifications.
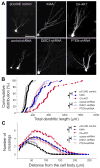
Figure 5. Role of KIAA1212 and AKT signaling in regulating dendritic development of newborn neurons in the adult brain
(A) Sample projected confocal images of GFP+ new neurons in the adult brain. Same as in Figure 4, GFP+ neurons with retrovirus-mediated genetic manipulation were examined at 14 dpi. Scale bar: 50 μm. (B) A summary of total dendritic length of GFP+ neurons at 14 dpi under different conditions. Shown is the cumulative plot with each symbol representing data from an individual GFP+ neuron from at least four animals for each condition. (*: P < 0.01, Kolmogorov-Smirnov test) (C) Analysis of dendritic complexity of GFP+ neurons using the Sholl analysis. The same sets of GFP+ neurons as in (B) were used. Values represent mean ± SEM (*: P < 0.01, student t-test)
Figure 6. Effects of pharmacological inhibition of mTOR on DISC1-dependent regulation of development of newborn neurons in the adult brain
(A) A schematic diagram of experimental design. Rapamycin (20 mg/kg body weight) or vehicle was i.p. injected. (B) Quantification of pS6 levels in GFP+ neurons under different experimental conditions. Similar to Figure 1C, the pS6 fluorescence intensity of individual GFP+DCX+ new neuron was normalized to the GFP-DCX+ immature new neurons in the same image. Values represent mean ± SEM (n = 42-49 cells from at least four animals; *: P < 0.01, ANOVA). (C-G) Development of new neurons in the dentate gyrus of the adult hippocampus. GFP+ neurons were examined at 14 dpi. Shown are summaries of the soma size (C) and number of primary dendrites (D), neuronal positioning (E), total dendritic length (F) and Sholl analysis (G) of GFP+ neurons under different conditions. Numbers associated with bar graph (in C) indicate the number of neurons examined from at least four animals under each condition. Values represent mean ± SEM (*: P <0.01, ANOVA).
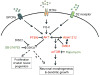
Figure 7. A model of DISC1 signaling pathways in regulating different aspects of adult hippocampal neurogenesis
RTKs: receptor tyrosine kinases; GPCRs: G protein–coupled receptors; D2 receptor: Dopamine type 2 (D2) receptor.
Comment in
- How DISC1 regulates postnatal brain development: girdin gets in on the AKT.
Porteous D, Millar K. Porteous D, et al. Neuron. 2009 Sep 24;63(6):711-3. doi: 10.1016/j.neuron.2009.09.017. Neuron. 2009. PMID: 19778497
Similar articles
- Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus.
Enomoto A, Asai N, Namba T, Wang Y, Kato T, Tanaka M, Tatsumi H, Taya S, Tsuboi D, Kuroda K, Kaneko N, Sawamoto K, Miyamoto R, Jijiwa M, Murakumo Y, Sokabe M, Seki T, Kaibuchi K, Takahashi M. Enomoto A, et al. Neuron. 2009 Sep 24;63(6):774-87. doi: 10.1016/j.neuron.2009.08.015. Neuron. 2009. PMID: 19778507 - Dixdc1 is a critical regulator of DISC1 and embryonic cortical development.
Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Singh KK, et al. Neuron. 2010 Jul 15;67(1):33-48. doi: 10.1016/j.neuron.2010.06.002. Neuron. 2010. PMID: 20624590 Free PMC article. - NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via Disrupted-In-Schizophrenia 1 (DISC1).
Namba T, Ming GL, Song H, Waga C, Enomoto A, Kaibuchi K, Kohsaka S, Uchino S. Namba T, et al. J Neurochem. 2011 Jul;118(1):34-44. doi: 10.1111/j.1471-4159.2011.07282.x. Epub 2011 May 19. J Neurochem. 2011. PMID: 21517847 Free PMC article. - DISC1-related signaling pathways in adult neurogenesis of the hippocampus.
Wu Q, Li Y, Xiao B. Wu Q, et al. Gene. 2013 Apr 15;518(2):223-30. doi: 10.1016/j.gene.2013.01.015. Epub 2013 Jan 24. Gene. 2013. PMID: 23353011 Review. - Disrupted-in-schizophrenia-1 (DISC1): a key susceptibility factor for major mental illnesses.
Sawamura N, Sawa A. Sawamura N, et al. Ann N Y Acad Sci. 2006 Nov;1086:126-33. doi: 10.1196/annals.1377.018. Ann N Y Acad Sci. 2006. PMID: 17185511 Review.
Cited by
- Disrupted-in-schizophrenia1 (DISC1) L100P mutation alters synaptic transmission and plasticity in the hippocampus and causes recognition memory deficits.
Cui L, Sun W, Yu M, Li N, Guo L, Gu H, Zhou Y. Cui L, et al. Mol Brain. 2016 Oct 12;9(1):89. doi: 10.1186/s13041-016-0270-y. Mol Brain. 2016. PMID: 27729083 Free PMC article. - Wnt7a regulates multiple steps of neurogenesis.
Qu Q, Sun G, Murai K, Ye P, Li W, Asuelime G, Cheung YT, Shi Y. Qu Q, et al. Mol Cell Biol. 2013 Jul;33(13):2551-9. doi: 10.1128/MCB.00325-13. Epub 2013 Apr 29. Mol Cell Biol. 2013. PMID: 23629626 Free PMC article. - The mechanism of Girdin in degenerative brain disease caused by high glucose stimulation.
Liu L, Zhang J, Han Y, Liu D. Liu L, et al. Front Endocrinol (Lausanne). 2022 Oct 3;13:892897. doi: 10.3389/fendo.2022.892897. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36329890 Free PMC article. - Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice.
Zhang H, Kang E, Wang Y, Yang C, Yu H, Wang Q, Chen Z, Zhang C, Christian KM, Song H, Ming GL, Xu Z. Zhang H, et al. Nat Commun. 2016 Jun 1;7:11773. doi: 10.1038/ncomms11773. Nat Commun. 2016. PMID: 27249678 Free PMC article. - Methylation pattern and mRNA expression of synapse-relevant genes in the MAM model of schizophrenia in the time-course of adolescence.
Khan AQ, Thielen L, Le Pen G, Krebs MO, Kebir O, Groh A, Deest M, Bleich S, Frieling H, Jahn K. Khan AQ, et al. Schizophrenia (Heidelb). 2022 Dec 8;8(1):110. doi: 10.1038/s41537-022-00319-8. Schizophrenia (Heidelb). 2022. PMID: 36481661 Free PMC article.
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Anai M, Shojima N, Katagiri H, Ogihara T, Sakoda H, Onishi Y, Ono H, Fujishiro M, Fukushima Y, Horike N, et al. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem. 2005;280:18525–18535. - PubMed
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. - PubMed
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD069184/HD/NICHD NIH HHS/United States
- R01 NS048271-06/NS/NINDS NIH HHS/United States
- R01 AG024984/AG/NIA NIH HHS/United States
- NS048271/NS/NINDS NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
- MH084018/MH/NIMH NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- NS047344/NS/NINDS NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- AG024984/AG/NIA NIH HHS/United States
- R01 NS048271-07/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous