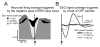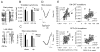Cortical firing and sleep homeostasis - PubMed (original) (raw)
Cortical firing and sleep homeostasis
Vladyslav V Vyazovskiy et al. Neuron. 2009.
Abstract
The need to sleep grows with the duration of wakefulness and dissipates with time spent asleep, a process called sleep homeostasis. What are the consequences of staying awake on brain cells, and why is sleep needed? Surprisingly, we do not know whether the firing of cortical neurons is affected by how long an animal has been awake or asleep. Here, we found that after sustained wakefulness cortical neurons fire at higher frequencies in all behavioral states. During early NREM sleep after sustained wakefulness, periods of population activity (ON) are short, frequent, and associated with synchronous firing, while periods of neuronal silence are long and frequent. After sustained sleep, firing rates and synchrony decrease, while the duration of ON periods increases. Changes in firing patterns in NREM sleep correlate with changes in slow-wave activity, a marker of sleep homeostasis. Thus, the systematic increase of firing during wakefulness is counterbalanced by staying asleep.
Conflict of interest statement
COI statement: All authors indicated no financial conflicts of interest.
Figures
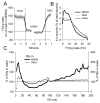
Figure 1. Cortical activity in sleep and waking
(A and B) Hypnogram, EEG traces from the right barrel cortex and corresponding electromyogram (EMG) in a representative rat during a 2-hour interval of undisturbed baseline starting at light onset (positivity is upward). (C) Average EEG power spectra in NREM sleep, REM sleep and waking (mean + SEM, n = 6 rats). Note high values of spectral power in the slow waves range (SWA, 0.5–4.0 Hz; grey bar) in NREM sleep. (D) Raw multiunit activity (MUA) recorded simultaneously in the same rat from a microwire array placed in the left barrel cortex (6 individual channels are shown). Note high tonic firing in waking and REM sleep, and OFF periods in NREM sleep. (E) Raster plots of spike activity for the same 6 channels shown in D (each vertical line is a spike). Note the close temporal relationship between OFF periods and the negative phase of EEG slow waves. Spike sorting was done according to a standard technique (see Experimental Procedures and Figure S1) and the recorded neural population was stable over time (Figure S2).
Figure 2. Effects of vigilance states on cortical neuronal firing
(A) Time course of mean firing rates during wake-NREM sleep and NREM-REM sleep transitions. Mean values (± SEM) represented as % of mean firing rates in NREM sleep (n=6 rats, 213 neurons). Note that firing rates decrease rapidly after the wake-NREM sleep transition and start increasing ~ 30 sec prior to the onset of REM sleep. (B) Distribution of 4-sec epochs in NREM sleep, REM sleep and waking as a function of mean firing rates (n=6 rats, 213 neurons). Note that high firing rates can be reached in NREM sleep close to the transition to REM sleep (panel A). (C) Distribution of ISIs in NREM sleep and REM sleep represented as % of the corresponding values in waking (mean values ± SEM, n=6 rats, 187 neurons).
Figure 3. EEG slow wave amplitude is related to the duration of the OFF periods
(A) The profile of average neuronal firing rates in NREM sleep aligned to the negative peak of the EEG slow wave (the peak is not shown, but is indicated by an arrow). Slow waves were subdivided in 3 categories based on their amplitude (low: 1–33%, intermediate: 34–66%, high: 67–100%) and the corresponding averages of neuronal activity were computed. Mean values (n = 6 rats). Note that high amplitude slow waves are associated with a larger suppression of neuronal activity. (B) Average EEG signal, aligned to the onset of OFF periods (arrow). All OFF periods were subdivided into 3 categories: 20–50 ms, 51–100 ms and >100 ms, and the corresponding averages of the EEG signal were computed (n = 6 rats). Note that the occurrence of OFF periods is consistently associated with negative waves in the surface EEG. Moreover, longer OFF periods are associated with larger slow waves.
Figure 4. Homeostatic changes in the patterns of neuronal activity during sleep
(A) NREM SWA (% of 12-hour baseline) and hypnogram of a 12-hour light period in one representative rat. (B) EEG and raster plots of neuronal activity in early and late NREM sleep in one representative rat. (C) Left: changes in incidence and duration of the ON periods during the light phase in one representative rat. Middle: Mean values (n = 6 rats) of incidence and duration of the ON periods shown for consecutive 4-hour intervals as percentage of the corresponding mean 12-hour value. (D) As in C, but for the OFF periods. Right panels: correlation between SWA (% of 12-hour light period mean) and incidence or duration of the ON and OFF periods in NREM sleep computed for consecutive NREM sleep episodes of the light period in one representative rat (significant correlations were found in all animals).
Figure 5. Decreased synchrony between individual neurons in late sleep
(A) Raster plots of spike activity in 6 channels during ON-OFF and OFF-ON transitions in early and late NREM sleep in one representative rat (each vertical bar represents one spike). Vertical dotted lines show the beginning and the end of the single OFF period depicted in the figure, while vertical thick lines indicate, for each neuron within the recorded population (6 neurons in this case), the average latency of their last and first spike from the onset of the OFF or ON periods, respectively (to assess their synchrony). (B) Neuronal synchrony at the ON-OFF and OFF-ON transition measured as 1/standard deviation (in ms) between the latencies of the last and first spike of each neuron from the onset of population OFF and ON transition, respectively (mean values + SEM, 125 neurons, n = 4 rats). Triangles, p<0.05. (C) Average slopes of the EEG slow waves. Triangles, p<0.05. (D) Average surface EEG slow waves aligned to their start point (ON-OFF transition) or their end point (OFF-ON transition). Mean slow waves (SEM, n=4 rats) are shown for the highest 50% and lowest 50% among all ON-OFF and OFF-ON transitions based on the synchrony between individual units (computed as in B). (E). Left: relationship between neuronal synchrony at ON-OFF or OFF-ON transitions and the corresponding slow wave slopes (% of mean). For each individual recording day (n = 4 rats, 2–5 days/rat) all ON-OFF and OFF-ON transitions were subdivided into 5 percentiles based on transition synchrony and the corresponding average slow wave slopes were computed. Right. Relationship between NREM SWA (0.5–4.0 Hz, % of 12-hour light period mean) and neuronal synchrony at the ON-OFF and OFF-ON transitions computed for the four 3-hour intervals of the light period (n=7 rats, 1–5 days/rat). Lines depict linear regression (Pearson).
Figure 6. Effects of sleep/waking history on firing rates
(A) Top left: SWA time course and corresponding hypnogram during a ~3-hour interval during the night, centered on a ~40-min long waking bout in one representative rat. Bottom left: examples of NREM ON periods before and after consolidated waking. Right: Average firing rates within the ON periods (mean values + SEM, 115 neurons, n = 4 rats). Triangle: p<0.05. (B) Top left: SWA and corresponding hypnogram during a 6-hour interval starting at light onset in one representative rat. Bottom left: examples of ON periods in early (within the first hour after lights on) and late (~ 5–6 hours after lights on) sleep. Right: Average firing rates within the ON periods in early and late sleep (mean values + SEM, 125 neurons, n = 4 rats). Triangle: p<0.05. Note that since different data sets contributed to panels (A) and (B), absolute firing rates values cannot be compared directly. (C) Relationship between NREM SWA (0.5–4.0 Hz, % of 12-hour light period mean) and neuronal firing rates within the ON periods (% of 12-hour light period mean) computed for the four 3-hour intervals of the light period (n=7 rats, 1–5 days/rat). Line depicts linear regression (Pearson). (D) Mean firing rates computed for NREM (including ON and OFF periods, 162 neurons, n=7 rats), waking (106 neurons, n=7 rats) and REM (136 neurons, n=7 rats) sleep in conditions of high and low sleep pressure. Triangles: p<0.05. To compare firing rates during waking, 4-sec epochs in high and low sleep pressure condition were equated based on EMG values. (E) Firing rates within the ON periods in NREM sleep before and after waking episodes lasting 5–25 min (n=7 rats). All waking episodes were subdivided into those without short sleep attempts (0% sleep) and those containing ~ 1–10% of NREM sleep. Mean values are shown as % of the mean between the bars. Triangle: p<0.05. (F) Firing rates in waking before and after sleep periods lasting <=60 min and consisting of > 70% of NREM sleep. Firing rates are shown separately for short sleep periods < 15 min and those longer than 15 min. Mean values are shown as % of the mean between the bars. Triangles: p<=0.05.
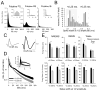
Figure 7. Effects of sleep-wake history on the firing rates of different neuronal subtypes
(A) Interspike intervals (ISIs) distribution for three representative neurons belonging to three major firing phenotypes, putative fast spiking (FS), putative regular spiking (RS) and putative intrinsically bursting (IB). Insets show the shortest ISIs (<20 ms). Note the different scales on the y-axes. (B) Distribution of individual neurons as a function of their spike width at ½ amplitude. All units were subdivided into two categories with narrow spikes and broad spikes (shaded areas). (C) Average spike waveforms corresponding to the two categories of narrow spike (<0.25 ms) and broad spike (>0.25 ms) units. (D) Interspike intervals (ISIs) distribution for neurons characterized by short action potential (<0.25 ms, putative inhibitory neurons, 45 neurons, n=6 rats) and for neurons characterized by long action potential (>0.25 ms, putative excitatory neurons, 38 neurons, n=6 rats). Mean values ±SEM. Inset highlights the differences between the two neuronal subtypes for the short ISIs (<20 ms). (E) Mean values (n=6–7 rats/group) of firing rates in NREM sleep, REM sleep and waking computed separately for the narrow spike and broad spike units for high and low sleep pressure conditions. Firing rates are shown as absolute values (top) and as % of the mean between the bars (bottom). Note the different scales on the y-axes. Triangles: p<0.05.
Figure 8. Effects of sleep deprivation on cortical firing
(A) SWA time course during the light period in baseline and after sleep deprivation (SDep) in one representative rat. Hypnogram from the same animal is shown below. (B) Time course of neuronal firing rates, and the number of long (>50 ms) and short (<20 ms) interspike intervals (ISIs) in waking during SDep (50 neurons, n= 5 rats). Mean values ± SEM shown as % of the value during the first hour of SDep. Asterisks: p<0.05. Inset: average firing rates during the first and fourth hour of SDep after equating the 4-sec epochs based on EMG values (mean values shown as % of the mean between the two bars). Triangle: p<0.05. (C) Number and duration of ON and OFF periods during the first hour of recovery after SDep (Rec), and corresponding time interval during baseline (BSL). Values are mean + SEM (n = 5 rats). Triangles, p<0.05. (D) Representative examples of ON periods (boxed) during baseline and recovery sleep in one rat. (E) Average firing rates within the ON periods during the first 1-hour interval after SDep and the corresponding time interval during baseline in NREM sleep (62 neurons, n=7 rats) and REM sleep (49 neurons, n =7 rats).
Comment in
- Resting our cortices by going DOWN to sleep.
Mignot E, Huguenard JR. Mignot E, et al. Neuron. 2009 Sep 24;63(6):719-21. doi: 10.1016/j.neuron.2009.09.008. Neuron. 2009. PMID: 19778500
Similar articles
- Local sleep in awake rats.
Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Vyazovskiy VV, et al. Nature. 2011 Apr 28;472(7344):443-7. doi: 10.1038/nature10009. Nature. 2011. PMID: 21525926 Free PMC article. - Why Does Sleep Slow-Wave Activity Increase After Extended Wake? Assessing the Effects of Increased Cortical Firing During Wake and Sleep.
Rodriguez AV, Funk CM, Vyazovskiy VV, Nir Y, Tononi G, Cirelli C. Rodriguez AV, et al. J Neurosci. 2016 Dec 7;36(49):12436-12447. doi: 10.1523/JNEUROSCI.1614-16.2016. J Neurosci. 2016. PMID: 27927960 Free PMC article. - The dynamics of cortical neuronal activity in the first minutes after spontaneous awakening in rats and mice.
Vyazovskiy VV, Cui N, Rodriguez AV, Funk C, Cirelli C, Tononi G. Vyazovskiy VV, et al. Sleep. 2014 Aug 1;37(8):1337-47. doi: 10.5665/sleep.3926. Sleep. 2014. PMID: 25083014 Free PMC article. - Cortical neuronal mechanisms of sleep homeostasis.
Vyazovskiy VV. Vyazovskiy VV. Zh Vyssh Nerv Deiat Im I P Pavlova. 2013 Jan-Feb;63(1):13-23. doi: 10.7868/s0044467713010176. Zh Vyssh Nerv Deiat Im I P Pavlova. 2013. PMID: 23697219 Review. - Electrophysiological correlates of sleep homeostasis in freely behaving rats.
Vyazovskiy VV, Cirelli C, Tononi G. Vyazovskiy VV, et al. Prog Brain Res. 2011;193:17-38. doi: 10.1016/B978-0-444-53839-0.00002-8. Prog Brain Res. 2011. PMID: 21854953 Free PMC article. Review.
Cited by
- Homeostatic sleep regulation in the absence of the circadian sleep-regulating component: effect of short light-dark cycles on sleep-wake stages and slow waves.
Szalontai Ö, Tóth A, Pethő M, Keserű D, Hajnik T, Détári L. Szalontai Ö, et al. BMC Neurosci. 2021 Feb 27;22(1):13. doi: 10.1186/s12868-021-00619-2. BMC Neurosci. 2021. PMID: 33639837 Free PMC article. - Spike-Based Functional Connectivity in Cerebral Cortex and Hippocampus: Loss of Global Connectivity Is Coupled to Preservation of Local Connectivity During Non-REM Sleep.
Olcese U, Bos JJ, Vinck M, Lankelma JV, van Mourik-Donga LB, Schlumm F, Pennartz CM. Olcese U, et al. J Neurosci. 2016 Jul 20;36(29):7676-92. doi: 10.1523/JNEUROSCI.4201-15.2016. J Neurosci. 2016. PMID: 27445145 Free PMC article. - Determinants of cortical synchrony.
Mongrain V, Warby SC. Mongrain V, et al. Sleep. 2012 Mar 1;35(3):309-10. doi: 10.5665/sleep.1680. Sleep. 2012. PMID: 22379234 Free PMC article. No abstract available. - Human cortical excitability increases with time awake.
Huber R, Mäki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Huber R, et al. Cereb Cortex. 2013 Feb;23(2):332-8. doi: 10.1093/cercor/bhs014. Epub 2012 Feb 7. Cereb Cortex. 2013. PMID: 22314045 Free PMC article. - Individual differences in white matter diffusion affect sleep oscillations.
Piantoni G, Poil SS, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJ, Van Der Werf YD. Piantoni G, et al. J Neurosci. 2013 Jan 2;33(1):227-33. doi: 10.1523/JNEUROSCI.2030-12.2013. J Neurosci. 2013. PMID: 23283336 Free PMC article.
References
- Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–693. - PubMed
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. - PubMed
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
- Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67:1018–1022. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous