Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding - PubMed (original) (raw)
Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding
J Chan et al. J Neurosci Methods. 1990 Aug.
Abstract
The limited success of immunogold labeling for pre-embedding immunocytochemistry of neuronal antigens is largely attributed to poor penetration of large (5-20 nm) colloidal gold particles. We examined the applicability of using silver intensification of 1 nm colloidal gold particles non-covalently bound to goat anti-rabbit immunoglobulin (1) for single labeling of a rabbit antiserum against the catecholamine synthesizing enzyme, tyrosine hydroxylase (TH), and (2) for immunogold localization of rabbit anti-TH simultaneously with immunoperoxidase labeling of a mouse monoclonal antibody against the opiate peptide, leucine-enkephalin (LE). Vibratome sections were collected from acrolein fixed brains of adult rats. These sections were immunolabeled without use of freeze-thawing or other methods that enhance penetration, but damage ultrastructure. By light microscopy, incubations in the silver intensifier (Intense M, Janssen) for less than 10 min at room temperature resulted in a brownish-red reaction product for TH. This product was virtually indistinguishable from that seen using diaminobenzidine reaction for detection of peroxidase immunoreactivity. Longer incubations produced intense black silver deposits that were more clearly distinguishable from the brown immunoperoxidase labeling. However, by light microscopy, the gold particles seen by electron microscopy were most readily distinguished from peroxidase reaction product with shorter silver intensification periods. The smaller size of gold particles with shorter periods of silver intensification also facilitated evaluation of labeling with respect to subcellular organelles. Detection of the silver product did not appear to be appreciably changed by duration of post-fixation in osmium tetroxide. In dual-labeled sections, perikarya and terminals exhibiting immunogold-silver labeling for TH were distinct from those containing immunoperoxidase labeling for LE. These results (1) define the conditions needed for optimal immunogold-silver labeling of antigens while maintaining the ultrastructural morphology in brain, and (2) establish the necessity for controlled silver intensification for light or electron microscopic differentiation of immunogold-silver and peroxidase reaction products and for optimal subcellular resolution.
Figures
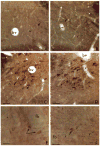
Fig. 1
Light micrographs showing immunogold labeling for tyrosine hydroxylase (TH). A–D: photomicrographs of 4 coronal sections through the medial nucleus of the solitary tract that were sequentially incubated in rabbit TH-antiserum and goat anti-rabbit IgG bound to 1 nm gold particles. A and B were silver intensified for 8 min while C and D were for 10 min. A and C were post-fixed for 20 min and B and D for 90 min in 2% osmium tetroxide. There is no notable diminution of the immunogold labeling with longer postfixation periods. E–F: photomicrographs from sections that were dually labeled for TH using immunogold and for LE by the ABC method through the caudate nucleus. Incubations in the silver intensifier were 12 min in E and 14 min in F. The brown peroxidase reaction for LE in the perikarya (large arrows) appears distinct from the black immunosilver labeled axons (small arrows) in F. bv, blood vessel; ts, tractus solitarius; fb, fiber bundle. Bar = 50 _μ_m.
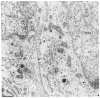
Fig. 2
Ultrastructural localization of immunogold silver labeling for TH in a neuronal perikaryon in the medial nucleus of the solitary tract with 4 min silver intensification. The gold particles are exclusively localized within the cytoplasm of the neuron whose nucleus (nuc 1) is shown. The particles appear preferentially associated with the outer saccule of the rough endoplasmic reticulum (rer). The cytoplasm of another cell with nucleus (nuc 2) is unlabeled. The tissue was post-fixed 20 min in 2% OsO4. Bar = 0.3 _μ_m.
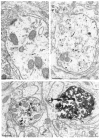
Fig. 3
Ultrastructural localization of immunogold silver labeling for TH in processes, presumably dendrites (den) in the medial nucleus of the solitary tract. Silver intensification for 2, 4 and 14 min in A–C, respectively. Small clusters of gold particles are barely detectable in A and assume larger and somewhat irregular shapes in B and C. The intensely labeled process to the right of the plate in C appears homogeneously electron dense resembling peroxidase immunoreactivity. Arrows in C indicate an even denser product resembling autoradiographic silver grains. A and B were from tissues incubated 20 min and C from tissue postfixed 60 min in 2% OsO4. Bar = 0.4 _μ_m throughout.
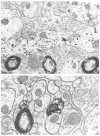
Fig. 4
Electron micrographs showing immunogold-silver labeling for TH in the medial nucleus of the solitary tract. A: after a 6 min silver intensification, individual, but irregular, silver clusters are seen in a longitudinally sectioned dendrite (den). This section was collected from the extreme outer surface of tissue postfixed 20 min in 2% OsO4. B: after a 14 min silver intensification, the silver product has coalesced to resemble peroxidase (see Fig. 5) labeling. This section was postfixed for 60 min in 2% OsO4 and was collected at a greater depth from the surface of the tissue than the section in A. ut = unlabeled axon terminal, arrows indicate regions of membrane for comparison of morphology obtained at different post-fixation times. Bars: A, 0.6 _μ_m; B, 0.3 _μ_m.
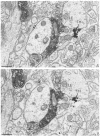
Fig. 5
Electron micrographs showing serial sections (A and B) immunolabeled with combined ABC for LE and gold-silver for TH. The tissue was postfixed 20 min in OsO4. Peroxidase reaction product is localized in axon terminals containing LE. Silver particles indicative of TH-labeling also are seen in an axon terminal (TH). Silver enhancement time was 6 min at room temperature. Bar = 0.5 _μ_m.
Similar articles
- Immunolabeling of retrogradely transported Fluoro-Gold: sensitivity and application to ultrastructural analysis of transmitter-specific mesolimbic circuitry.
Van Bockstaele EJ, Wright AM, Cestari DM, Pickel VM. Van Bockstaele EJ, et al. J Neurosci Methods. 1994 Nov;55(1):65-78. doi: 10.1016/0165-0270(94)90042-6. J Neurosci Methods. 1994. PMID: 7891464 - Ultrastructural basis for interactions between central opioids and catecholamines. I. Rostral ventrolateral medulla.
Milner TA, Pickel VM, Reis DJ. Milner TA, et al. J Neurosci. 1989 Jun;9(6):2114-30. doi: 10.1523/JNEUROSCI.09-06-02114.1989. J Neurosci. 1989. PMID: 2566665 Free PMC article. - Neuronal imaging with colloidal gold.
van den Pol AN. van den Pol AN. J Microsc. 1989 Jul;155(Pt 1):27-59. doi: 10.1111/j.1365-2818.1989.tb04297.x. J Microsc. 1989. PMID: 2671381 Review. - Colloidal gold and biotin-avidin conjugates as ultrastructural markers for neural antigens.
van den Pol AN. van den Pol AN. Q J Exp Physiol. 1984 Jan;69(1):1-33. doi: 10.1113/expphysiol.1984.sp002771. Q J Exp Physiol. 1984. PMID: 6201943 Review.
Cited by
- Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites.
Znamensky V, Akama KT, McEwen BS, Milner TA. Znamensky V, et al. J Neurosci. 2003 Mar 15;23(6):2340-7. doi: 10.1523/JNEUROSCI.23-06-02340.2003. J Neurosci. 2003. PMID: 12657693 Free PMC article. - Decreases in endogenous opioid peptides in the rat medullo-coerulear pathway after chronic morphine treatment.
Van Bockstaele EJ, Peoples J, Menko AS, McHugh K, Drolet G. Van Bockstaele EJ, et al. J Neurosci. 2000 Dec 1;20(23):8659-66. doi: 10.1523/JNEUROSCI.20-23-08659.2000. J Neurosci. 2000. PMID: 11102471 Free PMC article. - Changes in the subcellular distribution of NADPH oxidase subunit p47phox in dendrites of rat dorsomedial nucleus tractus solitarius neurons in response to chronic administration of hypertensive agents.
Glass MJ, Chan J, Frys KA, Oselkin M, Tarsitano MJ, Iadecola C, Pickel VM. Glass MJ, et al. Exp Neurol. 2007 Jun;205(2):383-95. doi: 10.1016/j.expneurol.2007.02.016. Epub 2007 Mar 3. Exp Neurol. 2007. PMID: 17418121 Free PMC article. - Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala.
Beckerman MA, Glass MJ. Beckerman MA, et al. Exp Neurol. 2011 Jan;227(1):149-58. doi: 10.1016/j.expneurol.2010.10.010. Epub 2010 Oct 21. Exp Neurol. 2011. PMID: 20970421 Free PMC article. - Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers.
Reyes BA, Carvalho AF, Vakharia K, Van Bockstaele EJ. Reyes BA, et al. Exp Neurol. 2011 Jul;230(1):96-105. doi: 10.1016/j.expneurol.2011.04.001. Epub 2011 Apr 16. Exp Neurol. 2011. PMID: 21515261 Free PMC article.
References
- Anden NE, Dahlstrom A, Fuxe K, Larsson K. Further evidence for the presence of nigrostiatal dopamine neurons in the rat. Am J Anat. 1965;116:329–334. - PubMed
- Andre D, Vuillon Cacciuttolo G, Bosler O. GABA nerve endings in the rat red nucleus combined detection with serotonin terminals using dual immunocytochemistry. Neuroscience. 1987;23:1095–1102. - PubMed
- Basbaum AI. A rapid and simple silver enhancement procedure for ultrastructural localization of the retrograde tracer WGA apo HRP-Av and its use in double-label studies with post-embedding immunocytochemistry. J Histochem Cytochem. 1989;37:1811–1815. - PubMed
- Broadwell RD, Cataldo AM. The neuronal endoplasmic reticulum: Its cytochemistry and contribution to the endomembrane system. J Histochem Cytochem. 1983;31:1077–1088. - PubMed
- Cuello AC. Monoclonal antibodies in neuroanatomical research. In: Chan-Palay V, Palay SL, editors. Cytochemical Methods in Neuroanatomy. Alan R. Liss, Inc; New York, NY: 1982. pp. 151–164.
Publication types
MeSH terms
Substances
Grants and funding
- HL18974/HL/NHLBI NIH HHS/United States
- R01 EY008055-09/EY/NEI NIH HHS/United States
- MH00078/MH/NIMH NIH HHS/United States
- R01 DA004600/DA/NIDA NIH HHS/United States
- R01 MH040342/MH/NIMH NIH HHS/United States
- P01 HL018974/HL/NHLBI NIH HHS/United States
- F32 NS007782-03/NS/NINDS NIH HHS/United States
- R37 MH040342/MH/NIMH NIH HHS/United States
- EY08055/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources