Actomyosin contractility controls cell surface area of oligodendrocytes - PubMed (original) (raw)
Actomyosin contractility controls cell surface area of oligodendrocytes
Angelika Kippert et al. BMC Cell Biol. 2009.
Abstract
Background: To form myelin oligodendrocytes expand and wrap their plasma membrane multiple times around an axon. How is this expansion controlled?
Results: Here we show that cell surface area depends on actomyosin contractility and is regulated by physical properties of the supporting matrix. Moreover, we find that chondroitin sulfate proteoglycans (CSPG), molecules associated with non-permissive growth properties within the central nervous system (CNS), block cell surface spreading. Most importantly, the inhibitory effects of CSPG on plasma membrane extension were completely prevented by treatment with inhibitors of actomyosin contractility and by RNAi mediated knockdown of myosin II. In addition, we found that reductions of plasma membrane area were accompanied by changes in the rate of fluid-phase endocytosis.
Conclusion: In summary, our results establish a novel connection between endocytosis, cell surface extension and actomyosin contractility. These findings open up new possibilities of how to promote the morphological differentiation of oligodendrocytes in a non-permissive growth environment. See related minireview by Bauer and ffrench-Constant: http://www.jbiol.com/content/8/8/78.
Figures
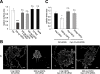
Figure 1
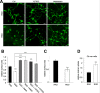
Integrins in cell surface expansion and endocytosis in Oli-neu cells. (A) Oli-neu cells were treated for 8 h with 100 nM RGD-peptide, 100 nM inactive RAD-peptide, 10 μM Y27632 and 50 μM blebbistatin (blebb) as indicated. Cells were stained with Alexa Fluor 488 conjugated wheat germ agglutinin (green). Scale bar, 10 μm. (B) Changes in relative surface area were quantified by image analysis as described in Material and Methods. (C) Changes in relative surface area shown for cells grown on fibronectin. (D) Quantitative analysis of dextran uptake (30 min) in Oli-neu cells treated with 100 nM RGD-peptide or inactive RAD-peptide for 8 h. Values represent means ± SEM (n > 70 cells, **p < 0,01; ***p < 0,001).
Figure 2
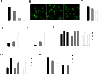
Matrix rigidity regulates cell surface area and endocytosis in Oli-neu cells. (A) Quantification of the elastic shear moduli of polyacrylamid gels on glass coverslips with varying amounts of bisacrylamid. Values represent means ± SD (n > 30 measurements). (B) Oli-neu cells were cultured for 1 d on polyacrylamid gels of different rigidities (using bisacrylamid varying from 0,5% to 0,01%) and analyzed for surface area differences by staining with Alexa Flour 488 conjugated wheat germ agglutinin (green). Scale bar, 10 μm. (C) Quantification of the surface area of Oli-neu cells on polyacrylamid gels of different rigidities (mean ± SEM; n > 100 cells, ***p < 0,001). (D+E) Cells were cultured for 1 d on polyacrylamid gels of different rigidities by varying the amount of bisacrylamid. Changes in the amount of dextran uptake ((D) 15 min and (E) 30 min) were quantified. Values represent the mean ± SEM (n > 60 cells from three independent experiments, ***p < 0,001). (F) Quantification of cell surface area of Oli-neu cells cultured on polyacrylamid gels for 1 d and treated for 10 h with 10 μM Y27632 or C3 transferase (mean ± SEM; n > 100 cells, *p < 0,05, **p < 0,01, ***p < 0,001). (G) Quantitative analysis of surface area changes of Oli-neu cells cultured for 1 d on polyacrylamid gels and treated with 50 μM blebbistatin (blebb) for 10 h (mean ± SEM; n > 60 cells, ***p < 0,001). (H) Oli-neu cells were cultured on polyacrylamid gels of different rigidities, treated with RGD-peptide or inactive RAD-peptide and changes in relative surface area were quantified. Values represent the mean ± SEM (n > 80 cells, *p < 0,05, **p < 0,01, ***p < 0,001).
Figure 3
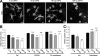
Matrix rigidity regulates cell surface area in primary oligodendrocytes. (A) Primary oligodendrocytes were cultured for 3-4 d on polyacrylamid gels of different rigidities and analyzed for the expansion of myelin membrane sheets by staining for MBP (green). Scale bar, 20 μm. (B) The relative sheet size of primary oligodendrocytes was quantified. Values represent the mean ± SEM (n > 60 cells, ***p < 0,001).
Figure 4
Chondroitin sulfate proteoglycans reduce cell surface area in Oli-neu cells. (A) Oli-neu cells were cultured for 12 h on coverslips coated with different concentrations of chondroitin sulfate proteoglycans (CSPG), stained with Alexa Fluor 488 conjugated wheat germ agglutinin (WGA) and analyzed for changes in cell surface area. Scale bar, 10 μm. (B) Quantification of the surface area of Oli-neu cells cultured on CSPG coated coverslips with or without 10 μM Y27632, C3 transferase or (C) 50 μM blebbistatin (means ± SEM; n > 80 cells, *p < 0,05, **p < 0,01, ***p < 0,001).
Figure 5
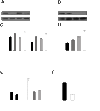
Myosin II regulates cell surface area and endocytosis in Oli-neu cells. (A+B) Western blot analysis of myosin (myo) IIA and IIB after siRNA knock down in Oli-neu cells as compared to actin as a loading control. (C) Control siRNA, siRNA directed against myosin (myo) IIA, myosin (myo) IIB or both were nucleofected into Oli-neu cells. The cells were subsequently cultured on CSPG coated coverslips and changes in cell surface area were quantified after 16 h (means ± SEM; n > 100 cells, n.s. not significant, ***p < 0,001). (D) Quantification of dextran uptake (30 min) in Oli-neu cells cultured on CSPG coated coverslips (means ± SEM; n > 100 cells, **p < 0,01, ***p < 0,001). (E) Quantification of dextran endocytosis in Oli-neu cells cultured on CSPG coated or control coverslips and treated with 10 μM Y27632 or 50 μM blebbistatin for 1 h (means ± SEM; n > 80 cells, *p < 0,05,***p < 0,001). (F) Quantitative analysis of the endocytosis of dextran in Oli-neu cells nucleofected with control siRNA or siRNA against both myosin IIA and IIB (means ± SEM; n > 80 cells from 2 independent experiments, ***p < 0,001). Scale bar 20 μm.
Figure 6
Inhibition of Myosin II promotes spreading of myelin-membrane sheets on CSPG. (A) Primary oligodendrocytes were cultured on CSPG coated or control coverslips treated with 10 μM Y27632 or 50 μM blebbistatin 2 h after seeding, stained for MBP and analyzed for changes in the size of myelin membrane sheets. Changes in cell surface area were quantified after 2-3 days. Values represent means ± SEM (n > 60 cells, n.s. not significant, ***p < 0,001). (B, C) Primary oligodendrocytes were nucleofected with siRNA directed against both myosin (myo) IIA and IIB or control (Ctrl) siRNA. The cells were subsequently cultured on CSPG coated or control coverslips and membrane sheet size was analyzed after 3 d. (C) Quantitative analysis of membrane sheet size (means ± SEM; n > 30 cells, *p < 0,05). (B) Primary oligodendrocytes stained with O1 antibody to visualize membrane sheets. Scale bar 20 μm.
Similar articles
- Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans.
Niederöst BP, Zimmermann DR, Schwab ME, Bandtlow CE. Niederöst BP, et al. J Neurosci. 1999 Oct 15;19(20):8979-89. doi: 10.1523/JNEUROSCI.19-20-08979.1999. J Neurosci. 1999. PMID: 10516316 Free PMC article. - Actomyosin-driven force patterning controls endocytosis at the immune synapse.
Kumari A, Pineau J, Sáez PJ, Maurin M, Lankar D, San Roman M, Hennig K, Boura VF, Voituriez R, Karlsson MCI, Balland M, Lennon Dumenil AM, Pierobon P. Kumari A, et al. Nat Commun. 2019 Jun 28;10(1):2870. doi: 10.1038/s41467-019-10751-7. Nat Commun. 2019. PMID: 31253773 Free PMC article. - Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ.
Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, McDonald JW. Pendleton JC, et al. Exp Neurol. 2013 Sep;247:113-21. doi: 10.1016/j.expneurol.2013.04.003. Epub 2013 Apr 12. Exp Neurol. 2013. PMID: 23588220 - The extracellular matrix: Focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies.
Pu A, Stephenson EL, Yong VW. Pu A, et al. Glia. 2018 Sep;66(9):1809-1825. doi: 10.1002/glia.23333. Epub 2018 Mar 30. Glia. 2018. PMID: 29603376 Review. - The oligodendrocyte precursor cell in health and disease.
Levine JM, Reynolds R, Fawcett JW. Levine JM, et al. Trends Neurosci. 2001 Jan;24(1):39-47. doi: 10.1016/s0166-2236(00)01691-x. Trends Neurosci. 2001. PMID: 11163886 Review.
Cited by
- Mechanotransduction assays for neural regeneration strategies: A focus on glial cells.
Marinval N, Chew SY. Marinval N, et al. APL Bioeng. 2021 Apr 30;5(2):021505. doi: 10.1063/5.0037814. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 33948526 Free PMC article. Review. - Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues.
Lourenço T, Paes de Faria J, Bippes CA, Maia J, Lopes-da-Silva JA, Relvas JB, Grãos M. Lourenço T, et al. Sci Rep. 2016 Feb 16;6:21563. doi: 10.1038/srep21563. Sci Rep. 2016. PMID: 26879561 Free PMC article. - Mechanical Strain Alters Cellular and Nuclear Dynamics at Early Stages of Oligodendrocyte Differentiation.
Makhija E, Jagielska A, Zhu L, Bost AC, Ong W, Chew SY, Shivashankar GV, Van Vliet KJ. Makhija E, et al. Front Cell Neurosci. 2018 Mar 6;12:59. doi: 10.3389/fncel.2018.00059. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29559894 Free PMC article. - Mechanical Strain Promotes Oligodendrocyte Differentiation by Global Changes of Gene Expression.
Jagielska A, Lowe AL, Makhija E, Wroblewska L, Guck J, Franklin RJM, Shivashankar GV, Van Vliet KJ. Jagielska A, et al. Front Cell Neurosci. 2017 Apr 20;11:93. doi: 10.3389/fncel.2017.00093. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28473753 Free PMC article. - Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles.
Bakhti M, Winter C, Simons M. Bakhti M, et al. J Biol Chem. 2011 Jan 7;286(1):787-96. doi: 10.1074/jbc.M110.190009. Epub 2010 Oct 26. J Biol Chem. 2011. PMID: 20978131 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources