Mitochondrial reactive oxygen species regulate hypoxic signaling - PubMed (original) (raw)
Review
Mitochondrial reactive oxygen species regulate hypoxic signaling
Robert B Hamanaka et al. Curr Opin Cell Biol. 2009 Dec.
Abstract
Physiological hypoxia results in a host of responses that include increased ventilation, constriction of the pulmonary artery, and a cellular transcriptional program that promotes glycolysis, angiogenesis, and erythropoiesis. Mitochondria are the primary consumers of cellular oxygen and have thus been speculated for years to be the site of cellular oxygen sensing. Many of the cellular responses to hypoxia are now known to be mediated by the production of reactive oxygen species at mitochondrial complex III. While the mechanism by which cytosolic oxidant concentration is increased during hypoxia is unknown, the importance of the maintenance of cellular oxygen supply requires further investigation into the role of ROS as hypoxia signaling molecules. The following is a brief overview of the current understanding of the role of mitochondrial-produced ROS in cellular oxygen signaling.
Figures
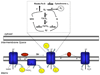
Figure 1. Mitochondrial generation of reactive oxygen species
Mitochondrial complexes I, II, and III produce superoxide. While complexes I and II only produce superoxide into the mitochondrial matrix, complex III can produce superoxide on both sides of the mitochondrial inner membrane in a process termed the Q-cycle. Complexes I and II donate two electrons to coenzyme Q, forming ubiquinol. At complex III, the first of these electrons is transferred by the Rieske iron-sulfur protein (RISP) to cytochrome _c_1, leaving the radical ubisemiquinone. Subsequently, ubisemiquinone transfers the second electron to cytochrome b. Ubisemiquinone formed at the Qo site can donate its electron directly to oxygen, producing superoxide.
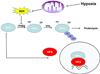
Figure 2. Hypoxia-induced mitochondrial ROS inhibit HIFα subunit turnover
Under normoxic conditions, HIFα subunits are hydroxylated on prolines by prolyl hydroxylases (PHDs). Hydroxylation tags HIFα for recognition by the von Hippel Lindau (VHL) tumor suppressor leading to ubiquitination and degradation of HIFα. During hypoxia, mitochondrial production of ROS inhibits the activity of PHDs allowing for stabilization of HIFα subunits and HIF-mediated transcription.
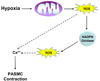
Figure 3. Hypoxia-induced mitochondrial ROS are required for elevation of cytosolic calcium and contraction of PASMCs
During hypoxia, mitochondrial production of ROS leads to activation of NADPH oxidases, further amplifying the ROS signal. The combined production of ROS at mitochondria and NADPH oxidase allows for elevation of cytosolic calcium and contraction of PASMCs.
Similar articles
- Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing.
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Guzy RD, et al. Cell Metab. 2005 Jun;1(6):401-8. doi: 10.1016/j.cmet.2005.05.001. Cell Metab. 2005. PMID: 16054089 - Mitochondrial complex III regulates hypoxic activation of HIF.
Klimova T, Chandel NS. Klimova T, et al. Cell Death Differ. 2008 Apr;15(4):660-6. doi: 10.1038/sj.cdd.4402307. Epub 2008 Jan 25. Cell Death Differ. 2008. PMID: 18219320 Review. - O2 sensing, mitochondria and ROS signaling: The fog is lifting.
Waypa GB, Smith KA, Schumacker PT. Waypa GB, et al. Mol Aspects Med. 2016 Feb-Mar;47-48:76-89. doi: 10.1016/j.mam.2016.01.002. Epub 2016 Jan 14. Mol Aspects Med. 2016. PMID: 26776678 Free PMC article. Review. - Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia.
Shohet RV, Garcia JA. Shohet RV, et al. J Mol Med (Berl). 2007 Dec;85(12):1309-15. doi: 10.1007/s00109-007-0279-x. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026917 Review. - Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells.
Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Waypa GB, et al. Circ Res. 2006 Oct 27;99(9):970-8. doi: 10.1161/01.RES.0000247068.75808.3f. Epub 2006 Sep 28. Circ Res. 2006. PMID: 17008601
Cited by
- From AKI to CKD: Maladaptive Repair and the Underlying Mechanisms.
Wang Z, Zhang C. Wang Z, et al. Int J Mol Sci. 2022 Sep 17;23(18):10880. doi: 10.3390/ijms231810880. Int J Mol Sci. 2022. PMID: 36142787 Free PMC article. Review. - Brown adipose tissue CoQ deficiency activates the integrated stress response and FGF21-dependent mitohormesis.
Chang CF, Gunawan AL, Liparulo I, Zushin PH, Vitangcol K, Timblin GA, Saijo K, Wang B, Parlakgül G, Arruda AP, Stahl A. Chang CF, et al. EMBO J. 2024 Jan;43(2):168-195. doi: 10.1038/s44318-023-00008-x. Epub 2024 Jan 11. EMBO J. 2024. PMID: 38212382 Free PMC article. - Potential Role of Cytochrome c and Tryptase in Psoriasis and Psoriatic Arthritis Pathogenesis: Focus on Resistance to Apoptosis and Oxidative Stress.
Chimenti MS, Sunzini F, Fiorucci L, Botti E, Fonti GL, Conigliaro P, Triggianese P, Costa L, Caso F, Giunta A, Esposito M, Bianchi L, Santucci R, Perricone R. Chimenti MS, et al. Front Immunol. 2018 Oct 30;9:2363. doi: 10.3389/fimmu.2018.02363. eCollection 2018. Front Immunol. 2018. PMID: 30429845 Free PMC article. Review. - Hypoxia Promotes Immune Evasion by Triggering β-Glucan Masking on the Candida albicans Cell Surface via Mitochondrial and cAMP-Protein Kinase A Signaling.
Pradhan A, Avelar GM, Bain JM, Childers DS, Larcombe DE, Netea MG, Shekhova E, Munro CA, Brown GD, Erwig LP, Gow NAR, Brown AJP. Pradhan A, et al. mBio. 2018 Nov 6;9(6):e01318-18. doi: 10.1128/mBio.01318-18. mBio. 2018. PMID: 30401773 Free PMC article. - Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability.
Janaszak-Jasiecka A, Siekierzycka A, Płoska A, Dobrucki IT, Kalinowski L. Janaszak-Jasiecka A, et al. Biomolecules. 2021 Jul 3;11(7):982. doi: 10.3390/biom11070982. Biomolecules. 2021. PMID: 34356605 Free PMC article. Review.
References
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. - PubMed
- Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. An excellent review explaining oxygen sensors in multiple cell types
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. An excellent overview of ROS production at various mitochondrial complexes
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM60472-10/GM/NIGMS NIH HHS/United States
- R01 CA123067-03/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 CA123067/CA/NCI NIH HHS/United States
- R01 GM060472/GM/NIGMS NIH HHS/United States
- T32 CA070085/CA/NCI NIH HHS/United States
- T32 CA070085-13/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources