A functional role for CCR6 on proallergic T cells in the gastrointestinal tract - PubMed (original) (raw)
A functional role for CCR6 on proallergic T cells in the gastrointestinal tract
Ana Belén Blázquez et al. Gastroenterology. 2010 Jan.
Abstract
Background & aims: CCL20 is a chemokine that regulates the homeostatic and inflammatory trafficking of leukocytes to the small intestine and regulates the development of the gastrointestinal lymphoid architecture. T cells expressing T helper cell (Th) 2 cytokines are critical for experimental food allergy, and we hypothesized that CCL20 is involved in the localization of these cells to the gut.
Methods: We evaluated the role of CCR6 in allergic diarrhea induced by sensitization and oral challenge with ovalbumin (OVA) using CCR6(+/+) and CCR6(-/-) mice.
Results: CCR6(-/-) mice were protected from OVA-induced diarrhea but surprisingly were not impaired in mastocytosis or allergen-specific immunoglobulin E. CCR6(-/-) mice were also protected from T cell-mediated diarrhea induced by anti-CD3 antibody. Allergic diarrhea was associated with an increased expression of Th2 cytokines within the intestinal mucosa that was significantly reduced in CCR6(-/-) mice. Inhibition of lymphocyte homing by treatment with FTY720 did not impair allergic diarrhea, indicating that reactivation of T cells could occur locally within the small intestine. Finally, T-cell transfer studies demonstrated that CCR6 was required both on the transferred T cells and in the recipient mouse to manifest allergic disease in the gastrointestinal tract.
Conclusions: These studies highlight a mast cell- and immunoglobulin E-independent role for CCR6-bearing T cells in the pathogenesis of gastrointestinal allergic disease.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no financial disclosures. The authors have no conflicts of interest to disclose.
Figures
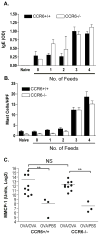
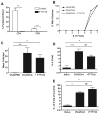
Figure 1. CCL20 is upregulated during allergic diarrhea and CCR6 is required for diarrhea symptoms
CCR6+/+ and CCR6−/− mice were sensitized and orally challenged with OVA. A. CCL20 mRNA expression in the jejunum of CCR6+/+ and CCR6−/− mice. Data are expressed as fold-changed compared to unchallenged controls. B. Immunostaining for CCL20 in the small intestine (bottom panel shows anti-CCL20 staining, top panel is isotype control). Note the dense immunoreactivity in the follicle-associated epithelium. C. Onset of symptoms (% of mice with visible diarrhea) after each feed was recorded. n = 33 (+/+) and 36 (−/−).
Figure 2. Allergen-induced Jejunal Mast Cells and serum IgE are CCR6-independent
CCR6+/+ and CCR6−/− mice were sensitized and with OVA. 4 mice were sacrificed after each feed of OVA (from 0 to 4). Top panel: Serum OVA-specific IgE. Middle panel: Jejunum mast cell counts per high power field (HPF). Bottom panel: MMCP-1 mRNA expression in jejunum from sacrificed after 4 (OVA/OVA) or 0 (OVA/PBS) feeds of OVA. ** p < 0.01, NS = not significant.
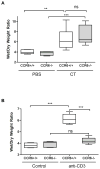
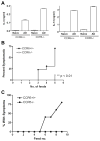
Figure 3. CCR6 is required for T cell-mediated, but not toxin-induced diarrhea
A: CCR6+/+ and CCR6−/− mice were fed 50 μg of cholera toxin or PBS as control. After 3 h, mice were euthanized and ligated loops were prepared from small intestine. Wet/dry weights were calculated as in methods. n = 3/group. B: CCR6+/+ and CCR6−/− mice were injected with 0.2 mg of anti-CD3 antibody or left un-injected as control. After 2 h, ligated loops were prepared as above. n = 7/group. ** p < 0.01; *** p < 0.001; ns = not significant.
Figure 4. Allergen-induced Th2 cytokine expression in the small intestine is impaired in CCR6 −/− mice
Left panels: CCR6+/+ and CCR6−/− mice were sensitized to OVA and challenged with OVA (OVA/OVA) or PBS (OVA/PBS) as control. Mice were euthanized within 1–2 h after symptom onset. (n= 10 samples/group) Right panels: Mice were sacrificed after each OVA feeding (4 mice/group). RNA was isolated from jejunum, and RT-PCR for IL-4 (top panel), IL-13 (middle panel) and IL-10 (bottom panel) was performed on individual samples (left panels) or pooled samples from the time course (right panels).
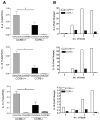
Figure 5. Th2 responses in the mesenteric lymph node are normal in CCR6 −/− mice
CCR6+/+ and CCR6−/− mice were sensitized to OVA and challenged with OVA (OVA/OVA) or PBS (OVA/PBS) as control. MLN cells were re-stimulated with OVA and IL-4, IL-5, IL-13, and IL-10 were measured in culture supernatants (Panel A). CFSE-labeled OVA-specific DO11.10 cells were transferred to naïve CCR6+/+ and CCR6−/− mice. Mice were fed 50 mg OVA (+OVA) or remained unfed as control (−OVA). After 96 h, T cell proliferation in the MLN was assessed by CFSE dilution (Panel B).
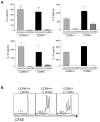
Figure 6. T cell reactivation occurs locally in the absence of homing from lymph nodes
Mice were treated with FTY720 daily beginning one day prior to beginning the OVA feeds. Efficacy of the FTY720 treatment was checked by flow cytometry of peripheral blood (A), which showed a near abolishment of circulating CD4 and CD8 T cells. FTY720 treatment had no effect on onset of OVA-induced diarrhea (B), jejunal mastocytosis (C), or jejunal IL-4 (D) or IL-13 (E) expression. Data are representative of two independent experiments, with a total of 10 mice per group.
Figure 7. CCR6 on T cells is necessary, but not sufficient, for allergic diarrhea
MLN cells from OVA-sensitized and fed (AD) CCR6+/+ or CCR6−/− mice were re-stimulated with OVA in vitro before transferring to naïve Balb/c mice. Mice were then fed with OVA every second day and diarrhea symptoms recorded. (A) The cytokine output of the transferred cells prior to transfer. (B) Symptoms of wild-type mice receiving primed CCR6+/+ or CCR6−/− T cells as above. (C) Donor cells were CCR6+/+, and recipients were CCR6+/+ or CCR6−/− as indicated.
Similar articles
- The C-C chemokine receptor 6 (CCR6) is crucial for Th2-driven allergic conjunctivitis.
Chung SH, Chang SY, Lee HJ, Choi SH. Chung SH, et al. Clin Immunol. 2015 Dec;161(2):110-9. doi: 10.1016/j.clim.2015.08.004. Epub 2015 Aug 22. Clin Immunol. 2015. PMID: 26307432 - Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy.
Kurashima Y, Kunisawa J, Higuchi M, Gohda M, Ishikawa I, Takayama N, Shimizu M, Kiyono H. Kurashima Y, et al. J Immunol. 2007 Aug 1;179(3):1577-85. doi: 10.4049/jimmunol.179.3.1577. J Immunol. 2007. PMID: 17641024 - IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10-Deficient Mice.
Polukort SH, Rovatti J, Carlson L, Thompson C, Ser-Dolansky J, Kinney SR, Schneider SS, Mathias CB. Polukort SH, et al. J Immunol. 2016 Jun 15;196(12):4865-76. doi: 10.4049/jimmunol.1600066. Epub 2016 May 6. J Immunol. 2016. PMID: 27183617 Free PMC article. - CC chemokine receptor 6 (CCR6) in the pathogenesis of systemic lupus erythematosus.
Lee AY, Körner H. Lee AY, et al. Immunol Cell Biol. 2020 Nov;98(10):845-853. doi: 10.1111/imcb.12375. Epub 2020 Aug 2. Immunol Cell Biol. 2020. PMID: 32634857 Review. - An immune paradox: how can the same chemokine axis regulate both immune tolerance and activation?: CCR6/CCL20: a chemokine axis balancing immunological tolerance and inflammation in autoimmune disease.
Comerford I, Bunting M, Fenix K, Haylock-Jacobs S, Litchfield W, Harata-Lee Y, Turvey M, Brazzatti J, Gregor C, Nguyen P, Kara E, McColl SR. Comerford I, et al. Bioessays. 2010 Dec;32(12):1067-76. doi: 10.1002/bies.201000063. Epub 2010 Oct 15. Bioessays. 2010. PMID: 20954179 Review.
Cited by
- Memory Generation and Re-Activation in Food Allergy.
Koenig JFE, Bruton K, Phelps A, Grydziuszko E, Jiménez-Saiz R, Jordana M. Koenig JFE, et al. Immunotargets Ther. 2021 Jun 9;10:171-184. doi: 10.2147/ITT.S284823. eCollection 2021. Immunotargets Ther. 2021. PMID: 34136419 Free PMC article. Review. - Relevant fecal microbes isolated from mice with food allergy elicited intestinal cytokine/chemokine network and T-cell immune responses.
Huang CH, Lu SY, Tsai WC. Huang CH, et al. Biosci Microbiota Food Health. 2020;39(4):234-242. doi: 10.12938/bmfh.2020-014. Epub 2020 Jul 4. Biosci Microbiota Food Health. 2020. PMID: 33117622 Free PMC article. - Modulation of the CCR6-CCL20 Axis: A Potential Therapeutic Target in Inflammation and Cancer.
Ranasinghe R, Eri R. Ranasinghe R, et al. Medicina (Kaunas). 2018 Nov 16;54(5):88. doi: 10.3390/medicina54050088. Medicina (Kaunas). 2018. PMID: 30453514 Free PMC article. Review. - Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector TH2 subsets.
Chiang D, Chen X, Jones SM, Wood RA, Sicherer SH, Burks AW, Leung DYM, Agashe C, Grishin A, Dawson P, Davidson WF, Newman L, Sebra R, Merad M, Sampson HA, Losic B, Berin MC. Chiang D, et al. J Allergy Clin Immunol. 2018 Jun;141(6):2107-2120. doi: 10.1016/j.jaci.2017.11.060. Epub 2018 Jan 31. J Allergy Clin Immunol. 2018. PMID: 29408715 Free PMC article. - Ana o 1 and Ana o 2 cashew allergens share cross-reactive CD4(+) T cell epitopes with other tree nuts.
Archila LD, Chow IT, McGinty JW, Renand A, Jeong D, Robinson D, Farrington ML, Kwok WW. Archila LD, et al. Clin Exp Allergy. 2016 Jun;46(6):871-83. doi: 10.1111/cea.12746. Clin Exp Allergy. 2016. PMID: 27129138 Free PMC article.
References
- Berin MC, Kiliaan AJ, Yang PC, Groot JA, Kitamura Y, Perdue MH. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J Immunol. 1998;161:2561–6. - PubMed
- Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–93. - PMC - PubMed
- Knight AK, Blazquez AB, Zhang S, Mayer LF, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007 - PubMed
- Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI044236/AI/NIAID NIH HHS/United States
- R21 DK071576-01/DK/NIDDK NIH HHS/United States
- AI044236/AI/NIAID NIH HHS/United States
- DK071576/DK/NIDDK NIH HHS/United States
- P01 AI044236/AI/NIAID NIH HHS/United States
- R21 DK071576/DK/NIDDK NIH HHS/United States
- U19 AI044236-100006/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical