Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis - PubMed (original) (raw)
. 2009 Nov;10(11):1178-84.
doi: 10.1038/ni.1791. Epub 2009 Sep 27.
Affiliations
- PMID: 19783988
- PMCID: PMC2898179
- DOI: 10.1038/ni.1791
Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis
Masako Murai et al. Nat Immunol. 2009 Nov.
Abstract
Regulatory T cells (T(reg) cells) that express the transcription factor Foxp3 suppress the activity of other cells. Here we show that interleukin 10 (IL-10) produced by CD11b(+) myeloid cells in recombination-activating gene 1-deficient (Rag1(-/-)) recipient mice was needed to prevent the colitis induced by transferred CD4(+)CD45RB(hi) T cells. In Il10(-/-)Rag1(-/-) mice, T(reg) cells failed to maintain Foxp3 expression and regulatory activity. The loss of Foxp3 expression occurred only in recipients with colitis, which indicates that the requirement for IL-10 is manifested in the presence of inflammation. IL-10 receptor-deficient (Il10rb(-/-)) T(reg) cells also failed to maintain Foxp3 expression, which suggested that host IL-10 acted directly on the T(reg) cells. Our data indicate that IL-10 released from myeloid cells acts in a paracrine manner on T(reg) cells to maintain Foxp3 expression.
Figures
Figure 1
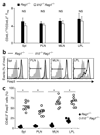
IL-10-deficient Treg cells prevent colitis. (a) Body weight of _Rag1_−/− mice given sorted _Il10_−/− or wild-type (WT) CD4+CD25+ Treg cells, together with CD4+CD45RBhi T cells, or of _Rag1_−/− mice given CD4+CD45RBhi T cells alone (control; None), presented relative to initial body weight. Data are pooled from two independent experiments with six mice each (error bars, s.d.). (b) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. NS, not significant. Data are pooled from at least two independent experiments.
Figure 2
_Rag1_−/− host IL-10 is required for Treg cell function. (a) Body weight of _Rag1_−/− or _Il10_−/−_Rag1_−/− hosts given CD4+CD45RBhi T cells plus sorted _Foxp3_gfp Treg cells, presented relative to initial body weight. Data are pooled from two independent experiments with ten mice each (error bars, s.d.). (b) Proximal colon of _Rag1_−/− and _Il10_−/−_Rag1_−/− mice at 6 weeks after the donor cell transfer described in a; sections are stained with hematoxylin and eosin. Original magnification, ×100; scale bars, 100 µm. Data are representative of one of three independent experiments. (c) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s _t_-test). Data are pooled from three independent experiments with a total of nine mice.
Figure 3
Foxp3 is downregulated in _Il10_−/−_Rag1_−/− recipients. (a) Composite ratios of CD45.1+ to CD45.2+ TCRβ+CD4+ cells in the spleen (Spl), PLNs, MLNs and LPL of _Rag1_−/− or _Il10_−/−_Rag1_−/− recipient mice at 6 weeks after injection of 4 × 105 CD4+CD45RBhi T cells derived from C57BL/6 (CD45.1+) mice, plus 1 × 105 _Foxp3_gfp (CD45.2+) Treg cells. (b) Foxp3 expression in the cells in a, gated on TCRβ+CD4+ CD45.2+ cells. Bracketed lines indicate the Foxp3− population. max, maximum. (c) Foxp3− cells in the gated TCRβ+CD4+ CD45.2+ populations in b. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s _t_-test). Data are pooled from three independent experiments with a total of nine mice (a (mean and s.d.) and c) or are representative of one of three independent experiments (b).
Figure 4
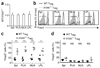
Loss of function by Treg cells from _Il10_−/−_Rag1_−/− recipients. (a,b) Flow cytometry of intracellular IFN-γ in CD4+CD45RBhi T cells derived from C57BL/6 (CD45.1+) mice transferred with _Foxp3_gfp (CD45.2+) Treg cells into _Rag1_−/− or _Il10_−/−_Rag1_−/− hosts; plots are gated on the TCRβ+CD4+ CD45.2− progeny of donor CD4+CD45RBhi T cells (a) and the TCRβ+CD4+ CD45.2+ progeny from donor _Foxp3_gfp + Treg cells (b), isolated from spleen (Spl), MLNs and LPL in mice at 6 weeks after donor cell injection and then stimulated with PMA and ionomycin. Numbers in plots indicate percent IFN-γ-producing cells. (c) Suppressive function in vitro of sorted TCRβ+CD4+ CD45.2+ cells from MLNs of _Rag1_−/− or _Il10_−/−_Rag1_−/− recipients of CD45.1+ CD4+CD45RBhi and CD45.2+ CD4+CD25+CD45RBlo Treg cell populations, cultured for 4 d together with CFSE-labeled CD45.1+ naive T cells; after stimulation of cultures, CFSE dilution was assessed by flow cytometry. Data are representative of one of three (a,b) or two (c) independent experiments.
Figure 5
_Il10rb_−/− Treg cells fail to prevent colitis. (a) Body weight of _Rag1_−/− recipients of C57BL/6 (CD45.1+) CD4+CD45RBhi T cells transferred together with wild-type or _Il10rb_−/− (CD45.2+) Treg cells, presented relative to initial body weight. Data are pooled from two independent experiments with a total of ten mice (error bars, s.d.). (b) Proximal colon of recipient mice at 6 weeks after injection of cells as described in a; sections are stained with hematoxylin and eosin. Original magnification, ×100; scale bars, 100 µm. Data are representative of one of three independent experiments. (c) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s _t_-test). Data are pooled from three independent experiments with a total of nine mice. (d) Foxp3 expression by cells isolated from the spleen, PLNs, MLNs and LPL of the recipient mice in a, with gating on TCRβ+CD4+CD45.2+ cells. Bracketed lines indicate the Foxp3− population. Data are representative of one of three independent experiments with a total of nine mice. (e) Foxp3− cells in the TCRβ+CD4+ CD45.2+ T lymphocyte populations described in d. Each symbol represents an individual mouse; small horizontal bars indicate the mean. *P < 0.001 (two-tailed Student’s _t_-test). Data are pooled from three independent experiments with a total of nine mice.
Figure 6
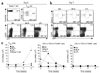
Foxp3 is lost ‘preferentially’ by _Il10rb_−/− Treg cells in mice with colitis. (a) Ratio of CD45.1+ to CD45.2+ CD90.1−CD4+TCRβ+ cells isolated from spleen, PLNs, MLNs and LPL of _Rag1_−/− recipients at 6 weeks after injection of 8 × 105 C57BL/6 (CD90.1+) CD4+CD45RBhi T cells, transferred with 2 × 104 wild-type (CD45.1+) Treg cells and 2 × 104 _Il10rb_−/− (CD45.2+) Treg cells. Data are pooled from two independent experiments with a total of four mice (mean and s.d.). (b) Flow cytometry of Foxp3 expression by cells isolated from a _Rag1_−/− recipient mouse as described in a, with gating on CD90.1−CD4+TCRβ+CD45.2− cells (wild-type Treg cells) or CD90.1−CD4+TCRβ+CD45.2+ cells (_Il10rb_−/− Treg cells). Bracketed lines indicate the Foxp3− population. Data are representative of one of two independent experiments. (c) Foxp3− cells in the CD90.1−CD4+TCRβ+CD45.2− (wild-type Treg) and CD90.1−CD4+TCRβ+CD45.2+ (_Il10rb_−/− Treg) populations isolated from _Rag1_−/− mice as described in a. Each symbol represents an individual mouse; small horizontal bars indicate the mean. Data are pooled from two independent experiments with a total of four mice. (d) Foxp3− cells in CD90.1−CD4+TCRβ+CD45.2− (wild-type Treg) or CD90.1−CD4+TCRβ+CD45.2+ (_Il10rb_−/− Treg) populations isolated from _Rag1_−/− recipients of 4 × 105 C57BL/6 (CD90.1+) CD4+CD45RBhi T cells, transferred together with 1 × 105 wild-type (CD45.1+) and 1 × 105 _Il10rb_−/− (CD45.2+) Treg cells. Each symbol represents an individual mouse; small horizontal bars indicate the mean. * P < 0.01; ** P < 0.001 (two-tailed Student’s _t_-test). Data are pooled from two independent experiments with a total of four mice.
Figure 7
Kinetics of IL-10 expression by Treg cells and host cells. (a) GFP+ cells in IL-10 reporter mice. Numbers adjacent to outlined areas (top row) indicate percent GFP+ cells among gated naive splenocytes (TCRβ+CD4+CD45RBhi) and Treg splenocytes (TCRβ+CD4+CD45RBloCD25+) from _Il10_gfp mice; numbers in top right quadrants (bottom row) indicate percent CD45+GFP+ cells in tissues from _Il10_gfp_Rag1_−/− mice. MFI, mean fluorescence intensity. (b) Flow cytometry of GFP+ cells in tissues 7 d after transfer of a mixture of CD45.1+ CD4+CD45RBhi T cells and CD45.2+ _Il10_gfp Treg cells (ratio, 4:1). Top row, Treg cells gated as CD45.2+ TCRβ+CD4+ cells; bottom row, gated TCRβ−CD4− nonlymphoid cells. Numbers adjacent to outlined areas and in top right quadrants indicate percent GFP+ cells. (c) Real-time PCR analysis of Il10 mRNA in sorted Treg cells (left), CD11b+ cells (middle) and CD11c+ dendritic cells (right) from various sites (keys) before transfer (0) or at 1, 2 and 6 weeks after transfer as in b. Data are from representative one of two independent experiments with six mice (a,b) or are pooled from two independent experiments with six mice (c; mean and s.d.).
Figure 8
IL-10-producing CD11b+ myeloid cells prevent the downregulation of Foxp3. Flow cytometry of Foxp3 expression 3 weeks after the injection of 5 × 106 _Rag1_−/− or _Il10_−/−_Rag1_−/− intestinal CD11c+CD11b+F4/80+ cells (a) or CD11c+CD11bF4/80− cells (b) into _Il10_−/−_Rag1_−/− recipients, transferred intravenously on days 0 and 7 (where ‘day 0’ is the day of T cell transfer) with 4 × 105 CD4+ CD45RBhi (CD45.1+) cells in the presence of 1 × 105 (CD45.2+) Treg cells from _Foxp3_gfp mice. Plots are gated on TCRβ+CD4+CD45.2+ splenocytes. Data are representative of one of two independent experiments with a total of three mice.
Comment in
- The gut feeling of Treg cells: IL-10 is the silver lining during colitis.
Unutmaz D, Pulendran B. Unutmaz D, et al. Nat Immunol. 2009 Nov;10(11):1141-3. doi: 10.1038/ni1109-1141. Nat Immunol. 2009. PMID: 19841645
Similar articles
- Novel Foxp3(-) IL-10(-) Regulatory T-cells Induced by B-Cells Alleviate Intestinal Inflammation in Vivo.
Shao TY, Hsu LH, Chien CH, Chiang BL. Shao TY, et al. Sci Rep. 2016 Sep 1;6:32415. doi: 10.1038/srep32415. Sci Rep. 2016. PMID: 27581189 Free PMC article. - The gut feeling of Treg cells: IL-10 is the silver lining during colitis.
Unutmaz D, Pulendran B. Unutmaz D, et al. Nat Immunol. 2009 Nov;10(11):1141-3. doi: 10.1038/ni1109-1141. Nat Immunol. 2009. PMID: 19841645 - IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells.
Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. Dardalhon V, et al. Nat Immunol. 2008 Dec;9(12):1347-55. doi: 10.1038/ni.1677. Epub 2008 Nov 9. Nat Immunol. 2008. PMID: 18997793 Free PMC article. - The Foxp3+ regulatory T cell: a jack of all trades, master of regulation.
Tang Q, Bluestone JA. Tang Q, et al. Nat Immunol. 2008 Mar;9(3):239-44. doi: 10.1038/ni1572. Nat Immunol. 2008. PMID: 18285775 Free PMC article. Review. - Roles for inflammation and regulatory T cells in colon cancer.
Erdman SE, Poutahidis T. Erdman SE, et al. Toxicol Pathol. 2010 Jan;38(1):76-87. doi: 10.1177/0192623309354110. Epub 2009 Dec 17. Toxicol Pathol. 2010. PMID: 20019355 Free PMC article. Review.
Cited by
- Postnatal supplementation with alarmins S100a8/a9 ameliorates malnutrition-induced neonate enteropathy in mice.
Perruzza L, Heckmann J, Rezzonico Jost T, Raneri M, Guglielmetti S, Gargari G, Palatella M, Willers M, Fehlhaber B, Werlein C, Vogl T, Roth J, Grassi F, Viemann D. Perruzza L, et al. Nat Commun. 2024 Oct 4;15(1):8623. doi: 10.1038/s41467-024-52829-x. Nat Commun. 2024. PMID: 39366940 Free PMC article. - Pravastatin prevents colitis-associated carcinogenesis by reducing CX3CR1high M2-like fibrocyte counts in the inflamed colon.
Hachiya K, Masuya M, Kuroda N, Yoneda M, Nishimura K, Shiotani T, Tawara I, Katayama N. Hachiya K, et al. Sci Rep. 2024 Oct 3;14(1):23021. doi: 10.1038/s41598-024-74215-9. Sci Rep. 2024. PMID: 39362935 Free PMC article. - Regulatory T cells: masterminds of immune equilibrium and future therapeutic innovations.
Ge J, Yin X, Chen L. Ge J, et al. Front Immunol. 2024 Sep 3;15:1457189. doi: 10.3389/fimmu.2024.1457189. eCollection 2024. Front Immunol. 2024. PMID: 39290699 Free PMC article. Review. - Immunomodulatory Effects of a Probiotic Mixture: Alleviating Colitis in a Mouse Model through Modulation of Cell Activation Markers and the Gut Microbiota.
Ryu HM, Islam SMS, Riaz B, Sayeed HM, Choi B, Sohn S. Ryu HM, et al. Int J Mol Sci. 2024 Aug 6;25(16):8571. doi: 10.3390/ijms25168571. Int J Mol Sci. 2024. PMID: 39201260 Free PMC article.
References
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. - PubMed
- Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. - PubMed
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. - PubMed
- Annacker O, et al. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008–3018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI050265-01/AI/NIAID NIH HHS/United States
- P01 DK46763/DK/NIDDK NIH HHS/United States
- R01 AI057992/AI/NIAID NIH HHS/United States
- P01 DK046763-170010/DK/NIDDK NIH HHS/United States
- R01 AI50265/AI/NIAID NIH HHS/United States
- R01 AI050265-08/AI/NIAID NIH HHS/United States
- P01 DK046763-180010/DK/NIDDK NIH HHS/United States
- R01 AI057992-02/AI/NIAID NIH HHS/United States
- R01 AI050265/AI/NIAID NIH HHS/United States
- P01 DK046763/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials