Differential effect of IL-27 on developing versus committed Th17 cells - PubMed (original) (raw)
Differential effect of IL-27 on developing versus committed Th17 cells
Mohamed El-behi et al. J Immunol. 2009.
Abstract
IL-27 counters the effect of TGF-beta+IL-6 on naive CD4(+) T cells, resulting in near complete inhibition of de novo Th17 development. In contrast, little is known about the effect of IL-27 on already differentiated Th17 cells. A better understanding of how IL-27 regulates these cells is needed to evaluate the therapeutic potential of IL-27 in Th17 cells-associated diseases. In this study, we show that IL-27 had surprisingly little effect on committed Th17 cells, despite its expression of a functional IL-27R. Contrary to de novo differentiation of Th17 cells, IL-27 did not suppress expression of retinoid-related orphan receptor (ROR)gammat or RORalpha in committed Th17 cells. Consistent with this finding, the frequency of committed Th17 cells and their cytokine secretion remained unaffected by IL-27. Both memory Th17 cells (CD4(+)CD25(-)CD62L(low)) that developed in vivo and encephalitogenic Th17 cells infiltrating the CNS of mice developing experimental autoimmune encephalomyelitis produced similar amounts of IL-17A when reactivated with IL-23 in the absence and presence of exogenous IL-27. Finally, IL-27 failed to suppress encephalitogenicity of Th17 cells in an adoptive transfer of experimental autoimmune encephalomyelitis. Analysis ex vivo of transferred Th17 cells in the spleen and CNS of recipient mice showed that cells retained similar phenotype irrespective of whether cells were treated or not with IL-27. Our data demonstrate that in contrast to inhibition of de novo differentiation of Th17 cells, IL-27 has little or no effect on committed Th17 cells. These findings indicate that therapeutic applications of IL-27 might have a limited efficacy in inflammatory conditions where aggressive Th17 responses have already developed.
Figures
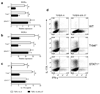
Figure 1. Suppressive effect of IL-27 on RORγt and RORα expression is dependent of STAT1 but independent of T-bet
CD4+ T cells from spleen of WT C57BL/6, T-bet−/−, and STAT1−/− mice were activated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β+IL-6 (± IL-27). 72 h after activation, cells were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (d). mRNA was extracted from cells cultivated in (d) and analyzed by real-time PCR for RORα (a) and RORγt (b) expression. IL-17A levels were measured by ELISA in the supernatants of cells activated during 72 h as described above (c). *p < 0.001. Data are representative of 3 experiments. (error bars, s.e.m).
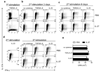
Figure 2. IL-27 does not suppress committed Th17 cells
Purified CD4+ T cells from spleens of C57BL/6 mice were activated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β+IL-6 (± IL-27), and anti-IFN-γ and anti-IL-4 antibodies (1st stimulation). Cells were activated 72 h later with PMA and ionomycin in the presence of GolgiPlug for 4 h and analyzed by flow cytometry for expression of IL-17A and IFN-γ (a). After the 1st stimulation, cells were rested 2 days in the presence of IL-2 and then reactivated with anti-CD3 and anti-CD28 antibodies (2nd stimulation) either during 3 days (b) or 6 days (c) in the presence of cytokine combinations indicated on each panel. Cells were then stimulated with PMA and ionomycin in the presence of GolgiPlug for the final 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression. Th17 cells that underwent a 2nd stimulation in the presence of IL-23 as described in (b) were rested for 2 days in the presence of IL-2 and then restimulated (3rd stimulation) with anti-CD3 and anti-CD28 antibodies, in the presence of cytokine combinations indicated on each panel. After 72 h cells were stimulated with PMA and ionomycin in the presence of GolgiPlug for the final 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (d). Cells undergoing a 2nd stimulation as described in (b) were pulsed with 1 µCi of [3H]thymidine for the last 18 h of culture, and thymidine incorporation was measured using a scintillation counter (e). Data are representative of two experiments (c, d, e) or five experiments (a, b).
Figure 3. IL-27 does not suppress cytokine production by committed Th17 cells
Concentrations of IL-17A (a), IL-22 (b), IL-21 (c) and IL-10 (d) in 72 h supernatants collected from cell cultures after 1st and 2nd stimulations as described in Figure 2. Changes in cytokine concentrations (%) when IL-27 was added to the culture compared to PBS are indicated above the bars. Data are representative of three experiments (error bars, s.e.m).
Figure 4. IL-27 does not suppress RORγt, RORα, and IL-23R expression in committed Th17 cells
Real-time PCR analysis of RORγt (a), RORα (b), and IL-23R (c) mRNA expression in differentiating Th17 cells and in committed Th17 cells after the 2nd stimulation. *p < 0.001. Data are representative of 2 experiments. (error bars, s.e.m).
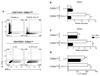
Figure 5. Committed Th17 cells express IL-27R and phosphorylate both STAT1 and STAT3 in response to IL-27
(a) Real-time PCR analysis of WSX-1 expression in differentiating Th17 cells and in committed Th17 cells after the 2nd stimulation. (b) Committed Th17 cells rested 2 days in the absence or presence of IL-2 (2 ng/ml) were either not stimulated (NS) or stimulated with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) antibodies (aCD3) in the presence of IL-6 (50 ng/ml) or IL-27 (50 ng/ml) for 30 min. Cells were then fixed, permeabilized and analyzed by flow cytometry for phosphorylated STAT1 (pSTAT1) and STAT3 (pSTAT3). *p < 0.001. Data are representative of 2 independent experiments. (error bars, s.e.m).
Figure 6. IL-27 does not suppress effector/memory Th17 cells that developed in vivo
Naive (CD4+CD25−CD62Lhigh) and memory T cells (CD4+CD25−CD62Llow) were sorted by flow cytometry and activated with anti-CD3 and anti-CD28 antibodies in the presence either of TGF-β+IL-6 (± IL-27) for naive cells, or IL-23 (± IL-27) for memory cells. All cultures were also supplemented with neutralizing anti-IFN-γ and anti-IL-4 antibodies. After 72 h, cells were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (a). mRNA was extracted from cells cultivated in (a) and analyzed by real-time PCR for RORγt (b) and RORα (c) expression. IL-17A levels were measured by ELISA in the supernatants of cells activated for 72 h as described above (d). *p < 0.001. Data are representative of 2 experiments. (error bars, s.e.m).
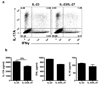
Figure 7. Myelin-specific Th17 cells that develop in vivo are resistant to suppression by IL-27
EAE was induced in C57BL/6 mice with MOG35–55 peptide. Brains and spinal cords were harvested at the peak of disease and mononuclear cells were isolated and stimulated for 3 days with MOG35–55 peptide in the presence of irradiated splenocytes and IL-23 (±IL-27). (a) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+ cells after stimulation with PMA, ionomycin, and GolgiPlug. (b) IL-17A, IFN-γ and IL-10 levels were measured by ELISA. Change in IL-17A concentration (%) when IL-27 was added to the culture is indicated above the bars.
Figure 8. IL-27 does not suppress encephalitogenicity of 2D2 Th17 cells in adoptive EAE model
2D2 Th17 were reactivated with MOG35–55 for 3 days in the presence of IL-23 (±IL-27). CD4+ T cells were enriched from culture and injected (7×106 cells/mouse) into 400 rad-irradiated C57BL/6 mice via the tail vein. Recipient mice received 200 ng of pertussis toxin on day 0 and 2 post-transfer. (a) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+ cells before transfer. (b) IL-17A, IFN-γ and IL-10 levels after the second stimulation of 2D2 cells were measured by ELISA. (d) Clinical scores of mice that received IL-23-stimulated or IL-23/IL-27-stimulated 2D2 Th17 cells. (c) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+Vβ11+ cells from spleens and isolated mononuclear cells from the CNS at day 32 post-transfer, after ex vivo stimulation with PMA, ionomycin, and GolgiPlug for 4 h. Change in IL-17A concentration (%) when IL-27 was added to the culture is indicated above the bars. Data are representative of two experiments. (error bars, s.e.m).
Similar articles
- IFN-beta inhibits human Th17 cell differentiation.
Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. Ramgolam VS, et al. J Immunol. 2009 Oct 15;183(8):5418-27. doi: 10.4049/jimmunol.0803227. Epub 2009 Sep 25. J Immunol. 2009. PMID: 19783688 - IFN-γ limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27.
Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, Weiner HL. Murugaiyan G, et al. J Immunol. 2012 Dec 1;189(11):5277-83. doi: 10.4049/jimmunol.1200808. Epub 2012 Nov 2. J Immunol. 2012. PMID: 23125412 Free PMC article. - Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis.
Lee K, Hwang S, Paik DJ, Kim WK, Kim JM, Youn J. Lee K, et al. Clin Exp Immunol. 2012 Oct;170(1):66-76. doi: 10.1111/j.1365-2249.2012.04637.x. Clin Exp Immunol. 2012. PMID: 22943202 Free PMC article. - IL-17 and Th17 Cells.
Korn T, Bettelli E, Oukka M, Kuchroo VK. Korn T, et al. Annu Rev Immunol. 2009;27:485-517. doi: 10.1146/annurev.immunol.021908.132710. Annu Rev Immunol. 2009. PMID: 19132915 Review. - Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation.
Chen Z, Laurence A, O'Shea JJ. Chen Z, et al. Semin Immunol. 2007 Dec;19(6):400-8. doi: 10.1016/j.smim.2007.10.015. Epub 2007 Dec 31. Semin Immunol. 2007. PMID: 18166487 Free PMC article. Review.
Cited by
- Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis.
Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Puel A, et al. Curr Opin Allergy Clin Immunol. 2012 Dec;12(6):616-22. doi: 10.1097/ACI.0b013e328358cc0b. Curr Opin Allergy Clin Immunol. 2012. PMID: 23026768 Free PMC article. Review. - Expression of IL-27, Th1 and Th17 in patients with aplastic anemia.
Du HZ, Wang Q, Ji J, Shen BM, Wei SC, Liu LJ, Ding J, Ma DX, Wang W, Peng J, Hou M. Du HZ, et al. J Clin Immunol. 2013 Feb;33(2):436-45. doi: 10.1007/s10875-012-9810-0. Epub 2012 Sep 30. J Clin Immunol. 2013. PMID: 23054344 - IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis.
Morrow KN, Coopersmith CM, Ford ML. Morrow KN, et al. Front Immunol. 2019 Aug 22;10:1982. doi: 10.3389/fimmu.2019.01982. eCollection 2019. Front Immunol. 2019. PMID: 31507598 Free PMC article. Review. - Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27.
Pot C, Apetoh L, Awasthi A, Kuchroo VK. Pot C, et al. Semin Immunol. 2011 Dec;23(6):438-45. doi: 10.1016/j.smim.2011.08.003. Epub 2011 Sep 3. Semin Immunol. 2011. PMID: 21893418 Free PMC article. Review. - The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases.
Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. Kuwabara T, et al. Mediators Inflamm. 2017;2017:3908061. doi: 10.1155/2017/3908061. Epub 2017 Feb 20. Mediators Inflamm. 2017. PMID: 28316374 Free PMC article. Review.
References
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Current Opinion in Immunology. 2007;19:281–286. - PubMed
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials