Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis - PubMed (original) (raw)
Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis
Alexandra Van Keymeulen et al. J Cell Biol. 2009.
Abstract
Merkel cells (MCs) are located in the touch-sensitive area of the epidermis and mediate mechanotransduction in the skin. Whether MCs originate from embryonic epidermal or neural crest progenitors has been a matter of intense controversy since their discovery >130 yr ago. In addition, how MCs are maintained during adulthood is currently unknown. In this study, using lineage-tracing experiments, we show that MCs arise through the differentiation of epidermal progenitors during embryonic development. In adults, MCs undergo slow turnover and are replaced by cells originating from epidermal stem cells, not through the proliferation of differentiated MCs. Conditional deletion of the Atoh1/Math1 transcription factor in epidermal progenitors results in the absence of MCs in all body locations, including the whisker region. Our study demonstrates that MCs arise from the epidermis by an Atoh1-dependent mechanism and opens new avenues for study of MC functions in sensory perception, neuroendocrine signaling, and MC carcinoma.
Figures
Figure 1.
MCs express epithelial markers during embryogenesis. (A) Immunostaining of K8 and K14 performed on skin sections shows the presence of MCs in E17 mice. (B) Immunostaining of K8 and K20 performed on skin sections of 1-d-old mice. (C) Confocal microscopy analysis of K8 and K14 immunostaining on skin sections of E17 embryo shows that some MCs coexpress K8 and the epidermal marker K14. (D) Immunostaining of K8 and P-cadherin on back skin sections at E17 shows the expression of P-cadherin, an epidermal progenitor marker in MCs. The inset is magnified (middle and right). Arrowheads point to MCs. (A, B, and D) Dashed lines delineate the basal layer of the epidermis. IFE, interfollicular epidermis. Bars, 20 µm.
Figure 2.
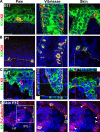
MCs originate from embryonic epidermal progenitors. (A–C) Confocal analysis of K8 and YFP immunostaining performed on vibrissae (A), paw (B), and skin (C) sections from K14-CRE/Rosa-YFP mice. (D–F) Immunofluorescence of YFP and Rab3c performed on skin sections from K14-CRE/Rosa-YFP mice. Arrowheads point to MCs. The inset in D is magnified (right). (G) Immunofluorescence of YFP performed on HF section from K14-CRE/Rosa-YFP mice (left) and bright field microscopy (right). (H) Immunofluorescence of YFP and NF200 performed on whisker section from K14-CRE/Rosa-YFP mice. Arrowheads point to nerves. DP, dermal papilla; Me, melanocytes; Ne, nerve. Bars, 20 µm.
Figure 3.
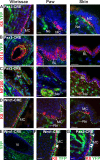
MCs do not originate from the neural crest progenitors. (A) Immunostaining of K8 and YFP performed on skin sections from Pax3-CRE/Rosa-YFP mice. (B) Immunofluorescence of K14 and YFP performed on skin sections from Pax3-CRE/Rosa-YFP mice showed that epidermal cells from the paw and back skin do not express YFP, whereas a mosaic expression of YFP could be seen in epidermal cells of the whisker. (C) Immunofluorescence of NF200 and YFP performed on skin sections from Pax3-CRE/Rosa-YFP mice. (D) Immunostaining of K8 and YFP performed on skin sections from Wnt1-CRE/Rosa-YFP mice. (E) Immunofluorescence of YFP from Wnt1-CRE/Rosa-YFP. (F and G) Immunofluorescence of K8 and YFP from Wnt1-CRE/Rosa-YFP (F) and Pax3-CRE/Rosa-YFP mice (G) showed that the percentage of MCs positive for YFP in vibrissae correlates with the degree of YFP chimerism in keratinocytes. Arrowheads point to K8– and YFP–double positive MCs. (A–E) Dashed lines delineate the basal layer (BL) of the epidermis. Ne, nerve; Vi, vibrissa. Bars, 20 µm.
Figure 4.
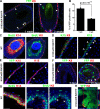
MC turnover during adult homeostasis is ensured by epidermal progenitors. (A) Immunostaining of K8 performed on vibrissa sections from K18-CREER/Rosa-YFP treated with 15 mg TAM and analyzed 1 and 3 wk after the last injection. (B) Quantification of YFP-positive cells in MCs (n = 2 mice per time point; error bars = SEM; p-value is from the paired Student's t test). (C) BrdU immunostaining in K14 (left)- and in K8 (right)-positive cells. (D) Immunostaining of K15 and K8 in the vibrissa follicle. (E) Immunostaining of K15 in vibrissa sections from K18-CREER/Rosa-YFP analyzed 1 wk after 15 mg TAM injection. (F) Immunostaining of K8 and YFP in vibrissa sections from K15-CREPR/Rosa-YFP analyzed 5 (left) and 21 d (right) after the administration of 2.5 mg RU486 per day. (G) BrdU immunostaining in K14 (left)- and in K8 (right)-positive cells. (H) Immunostaining of K8 performed on paw sections from K14-CREER/Rosa-YFP treated with TAM for 1 mo. (A and C–G) Dashed lines delineate the basal layer of the epidermis. (A and D–G) Arrowheads point to MCs (D, E, and G) and K8– and YFP–double positive MCs (A and F). WT, wild type. Bars, 20 µm.
Figure 5.
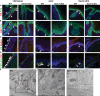
Conditional deletion of Atoh1 in embryonic epidermal progenitors results in the absence of MC specification. (A and B) Immunofluorescence of K14 and K8 (A) and K14 and K20 (B) performed in newborn wild-type (WT) and Atoh1 cKO mice shows the complete loss of MCs in Atoh1 cKO mice. Arrowheads point to MCs. (C) Immunofluorescence of K8 and Rab3c in 2-mo-old wild-type and Atoh1 cKO mice. Arrowheads point to K8– and Rab3c–double positive MCs. (D) FM1-43x administration to 1-mo-old mice resulted in the uptake of the fluorescent dye in MCs of wild-type mice but not in Atoh1 cKO mice. FM1-43x labeling is still observed in peripheral nerve endings in Atoh1 cKO mice. Arrowheads point to K8– and FM1-43x–double positive MCs. (E) Electron microscopy showed the absence of cells with electron-dense neurosecretory granules (Gr) contacting with nerve endings (Ne) in Atoh1 cKO. The inset is magnified (middle). (A–E) Dashed lines delineate the basal layer of the epidermis (A–D) or the MC (E). Bars, 20 µm.
Similar articles
- Ectopic Atoh1 expression drives Merkel cell production in embryonic, postnatal and adult mouse epidermis.
Ostrowski SM, Wright MC, Bolock AM, Geng X, Maricich SM. Ostrowski SM, et al. Development. 2015 Jul 15;142(14):2533-44. doi: 10.1242/dev.123141. Epub 2015 Jul 2. Development. 2015. PMID: 26138479 Free PMC article. - Mammalian Merkel cells are descended from the epidermal lineage.
Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Morrison KM, et al. Dev Biol. 2009 Dec 1;336(1):76-83. doi: 10.1016/j.ydbio.2009.09.032. Epub 2009 Sep 25. Dev Biol. 2009. PMID: 19782676 Free PMC article. - Notch pathway signaling in the skin antagonizes Merkel cell development.
Logan GJ, Wright MC, Kubicki AC, Maricich SM. Logan GJ, et al. Dev Biol. 2018 Feb 15;434(2):207-214. doi: 10.1016/j.ydbio.2017.12.007. Epub 2017 Dec 11. Dev Biol. 2018. PMID: 29241683 - The adult hair follicle: cradle for pluripotent neural crest stem cells.
Sieber-Blum M, Grim M. Sieber-Blum M, et al. Birth Defects Res C Embryo Today. 2004 Jun;72(2):162-72. doi: 10.1002/bdrc.20008. Birth Defects Res C Embryo Today. 2004. PMID: 15269890 Review. - Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results.
Halata Z, Grim M, Bauman KI. Halata Z, et al. Anat Rec A Discov Mol Cell Evol Biol. 2003 Mar;271(1):225-39. doi: 10.1002/ar.a.10029. Anat Rec A Discov Mol Cell Evol Biol. 2003. PMID: 12552639 Review.
Cited by
- Possible association between polyomaviruses and gastrointestinal complications: a narrative review.
Shadbash P, Hosseini SM, Shoraka S, Ghaemi A, Haghazali M, Mohebbi SR. Shadbash P, et al. Gastroenterol Hepatol Bed Bench. 2024;17(2):121-131. doi: 10.22037/ghfbb.v17i2.2796. Gastroenterol Hepatol Bed Bench. 2024. PMID: 38994506 Free PMC article. Review. - Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells.
Bardot ES, Valdes VJ, Zhang J, Perdigoto CN, Nicolis S, Hearn SA, Silva JM, Ezhkova E. Bardot ES, et al. EMBO J. 2013 Jul 17;32(14):1990-2000. doi: 10.1038/emboj.2013.110. Epub 2013 May 14. EMBO J. 2013. PMID: 23673358 Free PMC article. - Peripheral Mechanobiology of Touch-Studies on Vertebrate Cutaneous Sensory Corpuscles.
Cobo R, García-Piqueras J, García-Mesa Y, Feito J, García-Suárez O, Vega JA. Cobo R, et al. Int J Mol Sci. 2020 Aug 27;21(17):6221. doi: 10.3390/ijms21176221. Int J Mol Sci. 2020. PMID: 32867400 Free PMC article. Review. - Current In Vitro and In Vivo Models to Study MCPyV-Associated MCC.
Loke ASW, Lambert PF, Spurgeon ME. Loke ASW, et al. Viruses. 2022 Oct 7;14(10):2204. doi: 10.3390/v14102204. Viruses. 2022. PMID: 36298759 Free PMC article. Review. - Conversion of Sox2-dependent Merkel cell carcinoma to a differentiated neuron-like phenotype by T antigen inhibition.
Harold A, Amako Y, Hachisuka J, Bai Y, Li MY, Kubat L, Gravemeyer J, Franks J, Gibbs JR, Park HJ, Ezhkova E, Becker JC, Shuda M. Harold A, et al. Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):20104-20114. doi: 10.1073/pnas.1907154116. Epub 2019 Sep 16. Proc Natl Acad Sci U S A. 2019. PMID: 31527246 Free PMC article.
References
- Ben-Arie N., Hassan B.A., Bermingham N.A., Malicki D.M., Armstrong D., Matzuk M., Bellen H.J., Zoghbi H.Y. 2000. Functional conservation of atonal and Math1 in the CNS and PNS.Development. 127:1039–1048 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials