Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats - PubMed (original) (raw)
. 2009 Dec 29;164(4):1821-32.
doi: 10.1016/j.neuroscience.2009.09.046. Epub 2009 Sep 27.
K M Ramos, L C Loram, J Wieseler, P W Sholar, J J Kearney, M T Lewis, N Y Crysdale, Y Zhang, J A Harrison, S F Maier, K C Rice, L R Watkins
Affiliations
- PMID: 19788917
- PMCID: PMC2783248
- DOI: 10.1016/j.neuroscience.2009.09.046
Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats
M R Hutchinson et al. Neuroscience. 2009.
Abstract
Spinal cord microglial toll-like receptor 4 (TLR4) has been implicated in enhancing neuropathic pain and opposing morphine analgesia. The present study was initiated to explore TLR4-mediated pain modulation by intrathecal lipopolysaccharide, a classic TLR4 agonist. However, our initial study revealed that intrathecal lipopolysaccharide failed to induce low-threshold mechanical allodynia in naive rats, suggestive that TLR4 agonism may be insufficient to enhance pain. These studies explore the possibility that a second signal is required; namely, heat shock protein-90 (HSP90). This candidate was chosen for study given its known importance as a regulator of TLR4 signaling. A combination of in vitro TLR4 cell signaling and in vivo behavioral studies of pain modulation suggest that TLR4-enhancement of neuropathic pain and TLR4-suppression of morphine analgesia each likely require HSP90 as a cofactor for the effects observed. In vitro studies revealed that dimethyl sulfoxide (DMSO) enhances HSP90 release, suggestive that this may be a means by which DMSO enhances TLR4 signaling. While 2 and 100 microg lipopolysaccharide intrathecally did not induce mechanical allodynia across the time course tested, co-administration of 1 microg lipopolysaccharide with a drug that enhances HSP90-mediated TLR4 signaling now induced robust allodynia. In support of this allodynia being mediated via a TLR4/HSP90 pathway, it was prevented or reversed by intrathecal co-administration of a HSP90 inhibitor, a TLR4 inhibitor, a microglia/monocyte activation inhibitor (as monocyte-derived cells are the predominant cell type expressing TLR4), and interleukin-1 receptor antagonist (as this proinflammatory cytokine is a downstream consequence of TLR4 activation). Together, these results suggest for the first time that TLR4 activation is necessary but not sufficient to induce spinally mediated pain enhancement. Rather, the data suggest that TLR4-dependent pain phenomena may require contributions by multiple components of the TLR4 receptor complex.
Figures
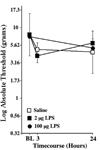
Figure 1. Lack of mechanical allodynia through 24hr after 2 or 100 µg LPS
Following von Frey testing prior to drug delivery (baseline; BL), rats were intrathecally administered either 0 (saline vehicle), 2 or 100 µg LPS. Von Frey responses were again recorded 3 and/or 24 hr later, with the 24 hr timepoint based on knowledge of the time course of effects known from the data illustrated in Figure 6 and Figure 7. n=6/group
Figure 2. Reversal of established chronic constriction injury (CCI)-induced mechanical allodynia by 2 inhibitors of HSP90
Panel A:
Reversal of CCI-induced mechanical allodynia by systemic geldanamycin. Following von Frey testing prior to CCI surgery (Pre-CCI), the sciatic nerve was loosely ligated, causing the fall in pain threshold recorded prior to systemic drug delivery (baseline; BL). Whereas subcutaneous (s.c.) vehicle produced no reliable reversal of allodynia (open squares), both 20 (filled squares) and 50 (filled triangles) µg/kg s.c. geldanamycin reduced allodynia at the 3 hr timepoint.
Panel B:
Reversal of CCI-induced mechanical allodynia by intrathecal 17-DMAG. Following von Frey testing prior to CCI surgery (Pre-CCI), the sciatic nerve was loosely ligated, causing the fall in pain threshold recorded prior to systemic drug delivery (baseline; BL). Whereas intrathecal (i.t.) vehicle produced no reliable reversal of allodynia (open squares), 10 µg i.t. 17-DMAG (filled circles) reduced allodynia at the 1 hr timepoint. n=6/group
Figure 3. Potentiation of intrathecal (i.t.) morphine by i.t. 17-DMAG, a HSP90 inhibitor
Following pre-drug baseline (BL) assessment on the Hargreaves test, rats were administered drugs and tested each 10 min for 175 min. While neither i.t. Vehicle + i.t. Vehicle (open squares) nor i.t. 17-DMAG (4 µg) + i.t. vehicle (filled squares) altered pain threshold, analgesia was induced by i.t. vehicle + i.t. morphine (15 µg), with a return to BL levels by 95–115 min. Analgesia produced by this dose of morphine was marked potentiated by coadministration of 4 µg 17-DMAG (filled circles), with a return to BL levels by 155–175 min later. n=6/group
Figure 4. Potentiation of TLR4 signaling in vitro by dimethyl sulfoxide (DMSO)
To test whether DMSO might enhance TLR4 signaling, HEK-TLR4 cells were incubated with 0 (media), 1, 10 or 100 ng/ml LPS, either 0 or 2% DMSO (each condition run in triplicate and either 0 (media), 0.00000001, 0.0000001, 0.000001, 0.00001, 0.0001, 0.001, 0.01, 0.1, 1, 10 or 100 ng/ml LPS combined with either 0, 0.1%, 1% or 2% DMSO in the media. Supernatants were collected and assayed for secreted alkaline phosphatase (SEAP) activity 24 hr later. SEAP activity, across LPS log doses are plotted for media (no DMSO; filled squares), 01% DMSO (open squares), 1% DMSO (open circles), and 2% DMSO (open diamonds). Each DMSO dose enhanced TLR4 signaling, as measured by SEAP activity. n=6/group
Figure 5. Blockade of DMSO-induced potentiation of TLR4 signaling in vitro by the HSP90 inhibitor, 17-DMAG
HEK-TLR4 cells were incubated with 0 (media), 1, 10 or 100 ng/ml lipopolysaccharide (LPS), either 0 or 2% DMSO (n= 3 replicates/condition), and either 0 (media; filled diamond), 0.01 (open diamond), 0.1 (open square), or 1 µg (open circle) 17-DMAG. Supernatants were collected and assayed for secreted alkaline phosphatase (SEAP) activity 24 hr later.
Panel A:
In the absence of DMSO, the HSP90 inhibitor had no effect except at the highest dose, which moderately suppressed TLR4 signaling.
Panel B:
In the presence of DMSO, TLR4 signaling was robustly enhanced in the absence of the HSP90 inhibitor (filled circle). All 3 17-DMAG doses (0.01 µg: open diamond; 0.1 µg: open square; 1 µg: open circle) markedly suppressed TLR4 signaling, returning SEAP activity to approximately the level obtained in the absence of DMSO (see Panel A). n=triplicate wells per condition
Figure 6. Induction of mechanical allodynia by intrathecal (i.t.) lipopolysaccharide (LPS) co-administered with i.t. dimethyl sulfoxide (DMSO)
After recording of pre-drug baseline (BL) withdrawal thresholds (von Frey test), rats were injected i.t. over lumbosacral spinal cord with either 1 µg LPS (open diamond), 4 µl DMSO (open square), or the combination of 1 µg LPS plus 4 µl DMSO (filled circle). Withdrawal thresholds were then retested 3 and 24 hr later. No between group differences were observed at BL or 3hr after i.t. injection. At 24 hr, only LPS+DMSO produced mechanical allodynia. n=6/group
Figure 7. Characterization of mechanical allodynia induced by intrathecal (i.t.) lipopolysaccharide (LPS) co-administered with i.t. dimethyl sulfoxide (DMSO): Effect of treatment with inhibitors of HSP90, TLR, and microglial activation prior to induction of allodynia
After recording of pre-drug baseline (BL) withdrawal thresholds (von Frey test), rats were injected i.t. over lumbosacral spinal cord with 1 µg LPS plus 4 µl DMSO as in Experiment 7. In addition, each rat was co-administered either vehicle (open square) or 17-DMAG (10 µg; filled square;
Panel A
), (+)-naloxone (20 µg; filled circle;
Panel B
), or minocycline (100 µg; filled diamond;
Panel C
). Withdrawal thresholds were then retested 24 hr later. All 3 test agents reliably reduced mechanical allodynia at 24 hr. n=6/group
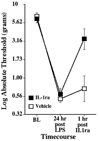
Figure 8. Characterization of mechanical allodynia induced by intrathecal LPS+DMSO: Effect of treatment with IL-1 inhibitor after induction of allodynia
After recording of pre-drug baseline (BL) withdrawal thresholds (von Frey test), rats were injected intrathecally over lumbosacral spinal cord with 1 µg LPS plus 4 µl DMSO. After confirming the development of mechanical allodynia 24hr later, rats were injected intrathecally with either vehicle (open square) or IL-1ra (100 µg; filled square). Withdrawal thresholds were then retested 1hr later. IL-1ra reversed the LPS+DMSO induced mechanical allodynia. n=6/group
Similar articles
- Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta.
Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Lewis SS, et al. Neuroscience. 2010 Jan 20;165(2):569-83. doi: 10.1016/j.neuroscience.2009.10.011. Neuroscience. 2010. PMID: 19833175 Free PMC article. - Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia.
Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Hutchinson MR, et al. Brain Behav Immun. 2008 Nov;22(8):1178-89. doi: 10.1016/j.bbi.2008.05.004. Epub 2008 Jul 2. Brain Behav Immun. 2008. PMID: 18599265 Free PMC article. - Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.
Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Zhang P, et al. Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review. - The role of heat shock protein 90 in regulating pain, opioid signaling, and opioid antinociception.
Streicher JM. Streicher JM. Vitam Horm. 2019;111:91-103. doi: 10.1016/bs.vh.2019.05.010. Epub 2019 Jul 15. Vitam Horm. 2019. PMID: 31421708 Review.
Cited by
- Neuraxial TNF and IFN-beta co-modulate persistent allodynia in arthritic mice.
Woller SA, Ocheltree C, Wong SY, Bui A, Fujita Y, Gonçalves Dos Santos G, Yaksh TL, Corr M. Woller SA, et al. Brain Behav Immun. 2019 Feb;76:151-158. doi: 10.1016/j.bbi.2018.11.014. Epub 2018 Nov 19. Brain Behav Immun. 2019. PMID: 30465880 Free PMC article. - Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF.
Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. Saito O, et al. Br J Pharmacol. 2010 Aug;160(7):1754-64. doi: 10.1111/j.1476-5381.2010.00811.x. Br J Pharmacol. 2010. PMID: 20649577 Free PMC article. - Pathological pain and the neuroimmune interface.
Grace PM, Hutchinson MR, Maier SF, Watkins LR. Grace PM, et al. Nat Rev Immunol. 2014 Apr;14(4):217-31. doi: 10.1038/nri3621. Epub 2014 Feb 28. Nat Rev Immunol. 2014. PMID: 24577438 Free PMC article. Review. - Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice.
Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Stokes JA, et al. J Neuroinflammation. 2013 Dec 9;10:148. doi: 10.1186/1742-2094-10-148. J Neuroinflammation. 2013. PMID: 24321498 Free PMC article. - Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy.
Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Li Y, et al. J Pain. 2014 Jul;15(7):712-25. doi: 10.1016/j.jpain.2014.04.001. Epub 2014 Apr 19. J Pain. 2014. PMID: 24755282 Free PMC article.
References
- Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. - PubMed
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. - PubMed
- Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. - PubMed
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA024044/DA/NIDA NIH HHS/United States
- K05 DA024044-01A1/DA/NIDA NIH HHS/United States
- R01 DA017670-04/DA/NIDA NIH HHS/United States
- K05 DA024044-02/DA/NIDA NIH HHS/United States
- DA015642/DA/NIDA NIH HHS/United States
- K02 DA015642/DA/NIDA NIH HHS/United States
- DA017670/DA/NIDA NIH HHS/United States
- R01 DA017670/DA/NIDA NIH HHS/United States
- DE017782/DE/NIDCR NIH HHS/United States
- R01 DA023132-02/DA/NIDA NIH HHS/United States
- R01 DA023132/DA/NIDA NIH HHS/United States
- K05 DA024044/DA/NIDA NIH HHS/United States
- R01 DA023132-02S2/DA/NIDA NIH HHS/United States
- R01 DA017670-05/DA/NIDA NIH HHS/United States
- R01 DE017782-03/DE/NIDCR NIH HHS/United States
- R01 DE017782-02/DE/NIDCR NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical