A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction - PubMed (original) (raw)
Randomized Controlled Trial
A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction
Hari K Parthasarathy et al. Eur J Heart Fail. 2009 Oct.
Abstract
Aims: To determine whether valsartan improves treadmill exercise time, in patients with symptomatic heart failure with a preserved ejection fraction (HFPEF), compared with placebo.
Methods and results: In this multicentred, double-blind, 14-week study, patients were randomized to receive valsartan (V) 80 mg or placebo (P) once daily on top of background medications. The dose of valsartan was force-titrated up to 320 mg. A total of 152 patients were randomized (V = 70, P = 82). Most patients had well-controlled hypertension (V = 91.2%, P = 89.0%) (mean baseline systolic BP approximately 130 mmHg) and >50% were receiving an angiotensin-converting enzyme inhibitor and/or beta-blocker (V = 57.4%, P = 54.9%). The mean ejection fraction at baseline was 70.48% in the placebo group (n = 64) and 71.52% in the valsartan group (n = 79). Valsartan had no significant effect on exercise time (primary variable), gas exchange variables, 6 min walk test distance, exertion-related symptoms, brain natriuretic peptide levels, echocardiographic parameters, or quality-of-life scores. Valsartan significantly lowered peak exercise systolic BP (-13.1 mmHg vs. placebo; P < 0.001) and improved ratings of perceived exertion (Borg score) (-0.69 vs. placebo; P = 0.008).
Conclusion: In this population, which predominantly included patients with well-controlled hypertension and symptomatic HFPEF, addition of valsartan did not increase exercise time within 14 weeks. However, valsartan 320 mg reduced blood pressure and improved symptoms of perceived exertion (Borg score) during exercise and was generally well-tolerated.
Figures
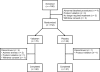
Figure 1
Patient disposition.
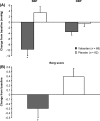
Figure 2
Least-squares mean changes from baseline to endpoint in (A) maximum systolic blood pressure (SBP) and maximum diastolic blood pressure (DBP) and (B) maximum Borg score. Error bars denote standard error of the mean. *P < 0.001 vs. placebo; †P = 0.008 vs. placebo.
Similar articles
- Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension.
Philipp T, Smith TR, Glazer R, Wernsing M, Yen J, Jin J, Schneider H, Pospiech R. Philipp T, et al. Clin Ther. 2007 Apr;29(4):563-80. doi: 10.1016/j.clinthera.2007.03.018. Clin Ther. 2007. PMID: 17617280 Clinical Trial. - Comparison of once-daily versus twice-daily dosing of valsartan in patients with chronic stable heart failure.
Anand IS, Deswal A, Kereiakes DJ, Purkayastha D, Zappe DH. Anand IS, et al. Vasc Health Risk Manag. 2010 Aug 9;6:449-55. Vasc Health Risk Manag. 2010. PMID: 20730060 Free PMC article. Clinical Trial. - Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial.
van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ. van der Bom T, et al. Circulation. 2013 Jan 22;127(3):322-30. doi: 10.1161/CIRCULATIONAHA.112.135392. Epub 2012 Dec 17. Circulation. 2013. PMID: 23247302 Clinical Trial. - Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial.
Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Solomon SD, et al. JACC Heart Fail. 2017 Jul;5(7):471-482. doi: 10.1016/j.jchf.2017.04.013. Epub 2017 Jun 26. JACC Heart Fail. 2017. PMID: 28662936 Review. - Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach.
Nixon RM, Müller E, Lowy A, Falvey H. Nixon RM, et al. Int J Clin Pract. 2009 May;63(5):766-75. doi: 10.1111/j.1742-1241.2009.02028.x. Int J Clin Pract. 2009. PMID: 19392925 Free PMC article. Review.
Cited by
- Treatment of Heart Failure With Preserved Ejection Fraction (HFpEF): the Phenotype-Guided Approach.
Silverman DN, Shah SJ. Silverman DN, et al. Curr Treat Options Cardiovasc Med. 2019 Apr 13;21(4):20. doi: 10.1007/s11936-019-0709-4. Curr Treat Options Cardiovasc Med. 2019. PMID: 30982123 Review. - Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction.
Martin N, Manoharan K, Thomas J, Davies C, Lumbers RT. Martin N, et al. Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD012721. doi: 10.1002/14651858.CD012721.pub2. Cochrane Database Syst Rev. 2018. PMID: 29952095 Free PMC article. Updated. Review. - Meta-analysis addressing the impact of cardiovascular-acting medication on peak oxygen uptake of patients with HFpEF.
Boulmpou A, Theodorakopoulou MP, Alexandrou ME, Boutou AK, Papadopoulos CE, Pella E, Sarafidis P, Vassilikos V. Boulmpou A, et al. Heart Fail Rev. 2022 Mar;27(2):609-623. doi: 10.1007/s10741-021-10207-5. Epub 2022 Jan 24. Heart Fail Rev. 2022. PMID: 35067835 - Comparing Sacubitril/Valsartan Against Sodium-Glucose Cotransporter 2 Inhibitors in Heart Failure: A Systematic Review and Network Meta-analysis.
Teo YN, Teo YH, Syn NL, Yoong CSY, Cheong AJY, Wee CF, Lim YC, Lee CH, Yeo TC, Chai P, Wong RCC, Lin W, Sia CH. Teo YN, et al. Clin Drug Investig. 2022 Jan;42(1):1-16. doi: 10.1007/s40261-021-01098-3. Epub 2021 Nov 19. Clin Drug Investig. 2022. PMID: 34797518 - The Current Focus of Heart Failure Clinical Trials.
Pothineni NV, Kattoor AJ, Kovelamudi S, Kenchaiah S. Pothineni NV, et al. J Card Fail. 2018 May;24(5):321-329. doi: 10.1016/j.cardfail.2018.02.006. Epub 2018 Mar 2. J Card Fail. 2018. PMID: 29482028 Free PMC article. Review.
References
- Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351:1097–1105. - PubMed
- Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. - PubMed
- MacFadyen RJ, MacLeod CM, Shiels P, Russell Smith W, MacDonald TM. Isolated diastolic heart failure as a cause of breathlessness in the community. the Arbroath study. Eur J Heart Fail. 2001;3:243–248. - PubMed
- Chinnaiyan KM, Alexander D, Maddens M, McCullough PA. Curriculum in cardiology:integrated diagnosis and management of diastolic heart failure. Am Heart J. 2007;153:189–200. - PubMed
- Aurigemma GP. Diastolic heart failure–a common and lethal condition by any name. N Engl J Med. 2006;355:308–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical