Isolation and partial purification of the Saccharomyces cerevisiae cytokinetic apparatus - PubMed (original) (raw)
Isolation and partial purification of the Saccharomyces cerevisiae cytokinetic apparatus
Brian A Young et al. Cytoskeleton (Hoboken). 2010 Jan.
Abstract
Cytokinesis is the process by which a cell physically divides in two at the conclusion of a cell cycle. In animal and fungal cells, this process is mediated by a conserved set of proteins including actin, type II myosin, IQGAP proteins, F-BAR proteins, and the septins. To facilitate biochemical and ultrastructural analysis of cytokinesis, we have isolated and partially purified the Saccharomyces cerevisiae cytokinetic apparatus. The isolated apparatus contains all components of the actomyosin ring for which we tested-actin, myosin heavy and light chain, and IQGAP-as well as septins and the cytokinetic F-BAR protein, Hof1p. We also present evidence indicating that the actomyosin rings associated with isolated cytokinetic apparati may be contractile in vitro, and show preliminary electron microscopic imaging of the cytokinetic apparatus. This first successful isolation of the cytokinetic apparatus from a genetically tractable organism promises to make possible a deeper understanding of cytokinesis.
Figures
Figure 1
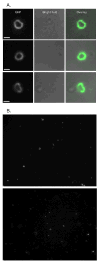
Release of intact Myo1-GFP rings from S. cerevisiae cells. A & B) Isolated Myo1-GFP rings from S. cerevisiae. Rings were concentrated for visualization by 13,000xg pelleting (See Figure 2). A) Comparisions of long pass GFP (GFPLP) fluorescent images and bright field images of close-up views of rings. Bright field images show that rings are not associated with cells. B) whole camera field views of rings. Bars ∼ 1 μm (1032 nm).
Figure 2
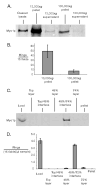
Concentration and enrichment of the budding yeast cytokinetic apparatus. A & B) Differential subcellular fractionation of lysate (cleared of unlysed cells by 300_xg_ spin). In A) 10 μg of each fraction was assayed for Myo1-GFP by quantitative immunoblotting. In B) the number of intact Myo1-GFP rings pelleted in 13,000_xg_ and 100,000_xg_ fractions was measured by spinning 15 μg clarified lysate onto slides and counting rings using fluorescence microscopy. C & D) Discontinuous sucrose density gradient fractionation. In C) 25 μl of each fraction was assayed for Myo1p by quantitative immunoblotting. In D) fractions were assayed by pelleting Myo1-GFP rings onto slides and counting microscopically. In both B & D) only morphologically distinct rings (crisp fluorescent outline, closed figures where the outline does not cross itself) were counted. “Fields” refers to ocular fields using a 100× objective (Area= 38,013 μm2). Error bars represent standard error of the mean of three measurements of ring numbers.
Figure 3
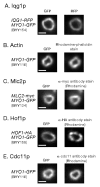
Known components of the cytokinetic apparatus are associated with Myo1-GFP rings purified by sucrose gradient. Rings were purified from cells containing markers indicated to the left (yeast strain number is in parenthesis). A) Iqg1p association was tested by RFP fluorescence imaging. B) Actin association was tested by rhodamine-phalloidin staining. C) Mlc2p-myc association was tested by α-myc antibody staining. D) Hof1p-HA association was tested by α-HA antibody staining. In some cases Hof1p staining throughout rings was less uniform compared to other components. E) Cdc11p (septin) association was tested with affinity purified α-Cdc11p antibody staining. Percentage of rings where component in question was associated: Iqg1p 96% (24/25); Actin 92% (23/25); Mlc2p 100%(15/15); Hof1p 93% (14/15); Cdc11p 100% (15/15). Only morphologically distinct rings (crisp fluorescent outline, closed figures where the outline does not cross itself) were tested for association. Bars ∼1 μm (1032 nm).
Figure 4
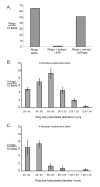
Evidence for functionality of isolated cytokinetic apparatus. A) Number of Myo1-GFP rings was measured for isolated rings alone, rings incubated with ATP and cytokinetic extracts; and rings incubated with cytokinetic extract and a highly active ATPase, apyrase, for ATP depletion. Consistent with in vitro ring contraction, ring titers declined in an energy-dependent fashion. Incubation time was in excess of that required for full contraction of rings in vivo. Rings were titered by pelleting onto slides and counted by fluorescence microscopy. “Fields” here refers to ocular fields using a 100× objective. Only morphologically distinct rings were counted. B) Size distribution of isolated Myo1-GFP rings in the absence of activation by ATP and cytokinetic extract (i.e. 0 minutes contraction time) was measured for rings incubated with cytokinetic extract & apyrase. C) Size distribution of isolated rings after incubation with ATP and cytokinetic extract for approximately half the time necessary for ring contraction in vivo. In B & C) ring sizes were determined by photographing pelleted rings and measuring them using ImageJ software. “Fields” here refers to CCD camera fields using a 100× objective. In both B & C) 3 sets of 15 fields were measured. Error bars represent the standard error of the mean.
Similar articles
- Isolation of Cytokinetic Actomyosin Rings from Saccharomyces cerevisiae and Schizosaccharomyces pombe.
Huang J, Mishra M, Palani S, Chew TG, Balasubramanian MK. Huang J, et al. Methods Mol Biol. 2016;1369:125-136. doi: 10.1007/978-1-4939-3145-3_10. Methods Mol Biol. 2016. PMID: 26519310 Free PMC article. - The Ringleaders: Understanding the Apicomplexan Basal Complex Through Comparison to Established Contractile Ring Systems.
Morano AA, Dvorin JD. Morano AA, et al. Front Cell Infect Microbiol. 2021 Apr 19;11:656976. doi: 10.3389/fcimb.2021.656976. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33954122 Free PMC article. Review. - Aim44p regulates phosphorylation of Hof1p to promote contractile ring closure during cytokinesis in budding yeast.
Wolken DM, McInnes J, Pon LA. Wolken DM, et al. Mol Biol Cell. 2014 Mar;25(6):753-62. doi: 10.1091/mbc.E13-06-0317. Epub 2014 Jan 22. Mol Biol Cell. 2014. PMID: 24451263 Free PMC article. - Biphasic targeting and cleavage furrow ingression directed by the tail of a myosin II.
Fang X, Luo J, Nishihama R, Wloka C, Dravis C, Travaglia M, Iwase M, Vallen EA, Bi E. Fang X, et al. J Cell Biol. 2010 Dec 27;191(7):1333-50. doi: 10.1083/jcb.201005134. Epub 2010 Dec 20. J Cell Biol. 2010. PMID: 21173112 Free PMC article. - The final cut: cell polarity meets cytokinesis at the bud neck in S. cerevisiae.
Juanes MA, Piatti S. Juanes MA, et al. Cell Mol Life Sci. 2016 Aug;73(16):3115-36. doi: 10.1007/s00018-016-2220-3. Epub 2016 Apr 16. Cell Mol Life Sci. 2016. PMID: 27085703 Free PMC article. Review.
Cited by
- Actin turnover maintains actin filament homeostasis during cytokinetic ring contraction.
Chew TG, Huang J, Palani S, Sommese R, Kamnev A, Hatano T, Gu Y, Oliferenko S, Sivaramakrishnan S, Balasubramanian MK. Chew TG, et al. J Cell Biol. 2017 Sep 4;216(9):2657-2667. doi: 10.1083/jcb.201701104. Epub 2017 Jun 27. J Cell Biol. 2017. PMID: 28655757 Free PMC article. - In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics.
Mishra M, Kashiwazaki J, Takagi T, Srinivasan R, Huang Y, Balasubramanian MK, Mabuchi I. Mishra M, et al. Nat Cell Biol. 2013 Jul;15(7):853-9. doi: 10.1038/ncb2781. Epub 2013 Jun 16. Nat Cell Biol. 2013. PMID: 23770677 - Actomyosin ring driven cytokinesis in budding yeast.
Meitinger F, Palani S. Meitinger F, et al. Semin Cell Dev Biol. 2016 May;53:19-27. doi: 10.1016/j.semcdb.2016.01.043. Epub 2016 Feb 1. Semin Cell Dev Biol. 2016. PMID: 26845196 Free PMC article. Review. - Isolation of Cytokinetic Actomyosin Rings from Saccharomyces cerevisiae and Schizosaccharomyces pombe.
Huang J, Mishra M, Palani S, Chew TG, Balasubramanian MK. Huang J, et al. Methods Mol Biol. 2016;1369:125-136. doi: 10.1007/978-1-4939-3145-3_10. Methods Mol Biol. 2016. PMID: 26519310 Free PMC article. - The Ringleaders: Understanding the Apicomplexan Basal Complex Through Comparison to Established Contractile Ring Systems.
Morano AA, Dvorin JD. Morano AA, et al. Front Cell Infect Microbiol. 2021 Apr 19;11:656976. doi: 10.3389/fcimb.2021.656976. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33954122 Free PMC article. Review.
References
- Balasubramanian MK, Bi E, Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol. 2004;14(18):R806–18. - PubMed
- Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322(1):21–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM042759/GM/NIGMS NIH HHS/United States
- R35 GM118149/GM/NIGMS NIH HHS/United States
- R37 GM042759/GM/NIGMS NIH HHS/United States
- R01 GM42759/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases