Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome - PubMed (original) (raw)
Comparative Study
Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome
Praveen Taneja et al. J Neurosci. 2009.
Abstract
Rett syndrome (RTT) is a neurodevelopmental disorder caused by loss-of-function mutations in the Methyl-CpG-binding protein-2 (MECP2) gene and is characterized by derangements in cognition, behavior, motor control, respiration and autonomic homeostasis, as well as seizures. Deficits in norepinephrine (NE) are thought to contribute to RTT pathogenesis, but little is known about how MeCP2 regulates function of noradrenergic neurons. We therefore characterized morphological, electrical, and neurochemical properties of neurons in the locus ceruleus (LC), the major source of noradrenergic innervation to the central neuraxis, in Mecp2 mutant mice. We found that MeCP2 null LC neurons are electrically hyperexcitable, smaller in size, and express less of the NE-synthesizing enzyme tyrosine hydroxylase (TH) compared with wild-type neurons. Increased excitability of mutant neurons is associated with reductions in passive membrane conductance and the amplitude of the slow afterhyperpolarization. Studies in Mecp2 heterozygotes, which are mosaic for the null allele, demonstrated that electrical hyperexcitability and reduced neuronal size are cell-autonomous consequences of MeCP2 loss, whereas reduced TH expression appears to reflect both cell-autonomous and non-autonomous influences. Finally, we found reduced levels of TH and norepinephrine in cingulate cortex, a forebrain target of the LC. Thus, genetic loss of MeCP2 results in a somewhat paradoxical LC neuron phenotype, characterized by both electrical hyperexcitability and reduced indices of noradrenergic function. Given the importance of the LC in modulating activity in brainstem and forebrain networks, we hypothesize that dysregulation of LC function in the absence of MeCP2 plays a key role in the pathophysiology of RTT.
Figures
Figure 1.
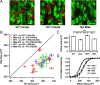
Cell-autonomous decrease in soma size of locus ceruleus neurons from null mice. A, Representative confocal images of neurons from the locus ceruleus of a WT female, a HET female, and a null male THGFP mouse (GFP in green) immunostained with MeCP2 (red) antibody. Scale bars, 20 μm. The MeCP2 antibody stained the WT neurons but not the null neurons. In HET females, approximately half of the neurons were stained by the MeCP2 antibody in an apparently random pattern. B, Average soma sizes of null (HET− for HET mice) versus WT (HET+ for HET mice) neurons for different age groups and mixed genetic background. The diagonal represents the identity line along which null soma sizes are identical to WT soma sizes. In all pairs, null neurons are smaller than WT neurons. There is no significant effect of age (for P26–P50), genetic background, or gender (within neurons from WT mice) on soma size. Average (C) and cumulative frequency distribution (D) of soma sizes (pooled data including all age groups, pure/mixed genetic background, and male/female mice for WT). In HET mice, average soma size of HET− neurons is smaller than that of HET+ neurons from the same animal (number of HET mice = 11). The decrease is similar to the decrease in size of neurons from null mice (n = 14) compared with WT mice (n = 21). The size of HET− neurons from HET mice is similar to neurons from null male mice, whereas the size of HET+ neurons from HET mice is similar to neurons from WT mice. BG, Background. ***p < 0.001, one-way ANOVA with post hoc Tukey's test.
Figure 2.
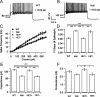
Cell-autonomous increase in excitability of locus ceruleus neurons from null mice. Representative firing of a neuron from WT (A) and null (B) mouse showing higher firing frequency for the latter for the same current injection. C, Average firing frequency for different current injections in neurons from WT and null mice and HET+ and HET− neurons from HET female mice (number of mice: WT = 6, null = 4, HET = 8; number of neurons: WT = 36, null = 34, HET+ = 29, HET− = 21). D, Slope of FI curves (linear fit between 90 and 300 pA) of neurons from WT, null, and HET female mice. In HET females, HET− neurons have a higher slope than HET+ neurons. The increase is similar to the increase in slope of neurons from null mice compared with WT mice. The slope of HET− neurons from HET mice is similar to neurons from null male mice, whereas the slope of HET+ neurons from HET mice is similar to neurons from WT mice. Average capacitance (E) and conductance (F) of neurons from WT, null, and HET female mice. HET− neurons from HET females have a smaller capacitance and conductance compared with HET+ neurons. The decrease in capacitance is similar to that seen in null mice relative to WT mice (decrease in conductance of neurons from null mice compared with that of WT mice is statistically significant by unpaired t test before including HET cells). The conductance and capacitance of HET− and HET+ neurons from HET mice is similar to that of neurons from null male mice and neurons from WT mice, respectively. *p < 0.05, ***p < 0.001, one-way ANOVA with post hoc Tukey's test.
Figure 3.
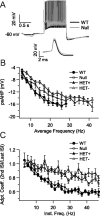
Neurons from the locus ceruleus of null mice have smaller psAHP and SFA compared with neurons from WT mice. A, Representative repetitive firing for neurons from WT and null mice at similar firing frequencies. Note the smaller psAHP in the recording from the null neuron. Inset, Single action potential at an extended timescale showing a slight increase in spike width in null neurons. B, Average psAHP at different average frequencies for neurons from WT, null, and HET female mice showing that HET− neurons from HET mice have a smaller psAHP than that of HET+ neurons (p < 0.01), just as the neurons from null mice have a smaller psAHP than that of WT neurons (p < 0.01). C, SFA coefficient (second ISI/last ISI) at different instantaneous firing frequencies (inverse of second ISI). HET− neurons from HET mice have a higher SFA coefficient (i.e., lower adaptation) than HET+ neurons (p < 0.01), just as neurons from null mice have a higher SFA coefficient than that of WT neurons (p < 0.01). Adpt. Coeff., Adaptation coefficient; Inst. Freq., instantaneous frequency. Differences were tested with two-way ANOVA and post hoc Tukey's test.
Figure 4.
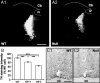
Reduced tyrosine hydroxylase protein level in the locus ceruleus and cingulate cortex of P35 null mice. A, Representative photomicrographs showing fluorescent TH immunostaining in the LC of P35 WT (A1) and null (A2) mice. B, Densitometric analysis revealed that P35 HET− LC neurons (n = 75) in HET females exhibit reduced TH staining compared with HET+ neurons (n = 105) in the same animals. In addition, the intensity of TH staining in P35 HET+ LC neurons in HET females is reduced compared with neurons in P35 WT females (n = 114). Pictures correspond to interaural coordinates −1.72 mm (Paxinos and Franklin, 2001). Scale bar, 200 μm. C, Representative photomicrographs of TH immunostained varicose neuronal processes (dark profiles) in the cingulate cortex of P35 WT (C1) and null (C2) mice, depicting decreased density of catecholaminergic processes in the cortex of null mice compared with WT. Scale bar, 250 μm. IV, Fourth ventricle; Cb, Cerebellum; cc, corpus callosum.
Figure 5.
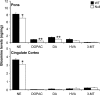
Biogenic amine content is significantly decreased in the pons and cingulate cortex of P35 null mice. HPLC analysis of bioamine levels in pons and cingulate cortex samples from WT (black bars) and null (white bars) mice. n = 6 mice; *p < 0.05, **p < 0.01, unpaired t test. DOPAC, Dihydroxyphenylacetic acid; DA, dopamine; HVA, homovanillic acid; 3MT, 3-methoxytyramine.
Similar articles
- Effects of early-life exposure to THIP on brainstem neuronal excitability in the Mecp2-null mouse model of Rett syndrome before and after drug withdrawal.
Zhong W, Johnson CM, Cui N, Oginsky MF, Wu Y, Jiang C. Zhong W, et al. Physiol Rep. 2017 Jan;5(2):e13110. doi: 10.14814/phy2.13110. Physiol Rep. 2017. PMID: 28108647 Free PMC article. - An optogenetic mouse model of rett syndrome targeting on catecholaminergic neurons.
Zhang S, Johnson CM, Cui N, Xing H, Zhong W, Wu Y, Jiang C. Zhang S, et al. J Neurosci Res. 2016 Oct;94(10):896-906. doi: 10.1002/jnr.23760. Epub 2016 Jun 18. J Neurosci Res. 2016. PMID: 27317352 Free PMC article. - Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome.
Oginsky MF, Cui N, Zhong W, Johnson CM, Jiang C. Oginsky MF, et al. Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C508-20. doi: 10.1152/ajpcell.00035.2014. Epub 2014 Jul 9. Am J Physiol Cell Physiol. 2014. PMID: 25009110 Free PMC article. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development.
Ribeiro MC, MacDonald JL. Ribeiro MC, et al. Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644. Epub 2020 Jan 2. Brain Res. 2020. PMID: 31904347 Free PMC article. Review.
Cited by
- The antitussive cloperastine improves breathing abnormalities in a Rett Syndrome mouse model by blocking presynaptic GIRK channels and enhancing GABA release.
Johnson CM, Cui N, Xing H, Wu Y, Jiang C. Johnson CM, et al. Neuropharmacology. 2020 Oct 1;176:108214. doi: 10.1016/j.neuropharm.2020.108214. Epub 2020 Jul 3. Neuropharmacology. 2020. PMID: 32622786 Free PMC article. - Pontine norepinephrine defects in Mecp2-null mice involve deficient expression of dopamine beta-hydroxylase but not a loss of catecholaminergic neurons.
Zhang X, Su J, Rojas A, Jiang C. Zhang X, et al. Biochem Biophys Res Commun. 2010 Apr 2;394(2):285-90. doi: 10.1016/j.bbrc.2010.02.156. Epub 2010 Mar 1. Biochem Biophys Res Commun. 2010. PMID: 20193660 Free PMC article. - Excitation and Inhibition Imbalance in Rett Syndrome.
Li W. Li W. Front Neurosci. 2022 Feb 18;16:825063. doi: 10.3389/fnins.2022.825063. eCollection 2022. Front Neurosci. 2022. PMID: 35250460 Free PMC article. Review. - Breathing Abnormalities During Sleep and Wakefulness in Rett Syndrome: Clinical Relevance and Paradoxical Relationship With Circulating Pro-oxidant Markers.
Leoncini S, Signorini C, Boasiako L, Scandurra V, Hayek J, Ciccoli L, Rossi M, Canitano R, De Felice C. Leoncini S, et al. Front Neurol. 2022 Mar 29;13:833239. doi: 10.3389/fneur.2022.833239. eCollection 2022. Front Neurol. 2022. PMID: 35422749 Free PMC article. - Negative Allosteric Modulation of mGluR5 Partially Corrects Pathophysiology in a Mouse Model of Rett Syndrome.
Tao J, Wu H, Coronado AA, de Laittre E, Osterweil EK, Zhang Y, Bear MF. Tao J, et al. J Neurosci. 2016 Nov 23;36(47):11946-11958. doi: 10.1523/JNEUROSCI.0672-16.2016. J Neurosci. 2016. PMID: 27881780 Free PMC article.
References
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–335. - PubMed
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. - PubMed
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. - PubMed
- Bauman ML, Kemper TL, Arin DM. Pervasive neuroanatomic abnormalities of the brain in three cases of Rett's syndrome. Neurology. 1995;45:1581–1586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD015052/HD/NICHD NIH HHS/United States
- R01 NS057398/NS/NINDS NIH HHS/United States
- R01 NS061340/NS/NINDS NIH HHS/United States
- P30 HD15052/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases