Clinical implications of KRAS mutations in lung cancer patients treated with tyrosine kinase inhibitors: an important role for mutations in minor clones - PubMed (original) (raw)
Multicenter Study
. 2009 Oct;11(10):1084-92.
doi: 10.1593/neo.09814.
Michele Milella, Lara Felicioni, Federico Cappuzzo, Luciana Irtelli, Maela Del Grammastro, Mariagrazia Sciarrotta, Sara Malatesta, Carmen Nuzzo, Giovanna Finocchiaro, Bruno Perrucci, Donatella Carlone, Alain J Gelibter, Anna Ceribelli, Andrea Mezzetti, Stefano Iacobelli, Francesco Cognetti, Fiamma Buttitta
Affiliations
- PMID: 19794967
- PMCID: PMC2745674
- DOI: 10.1593/neo.09814
Multicenter Study
Clinical implications of KRAS mutations in lung cancer patients treated with tyrosine kinase inhibitors: an important role for mutations in minor clones
Antonio Marchetti et al. Neoplasia. 2009 Oct.
Abstract
Mutations inducing resistance to anti-epidermal growth factor receptor (EGFR) therapy may have a clinical impact even if present in minor cell clones which could expand during treatment. We tested this hypothesis in lung cancer patients treated with tyrosine kinase inhibitors (TKIs). Eighty-three patients with lung adenocarcinoma treated with erlotinib or gefitinib were included in this study. The mutational status of KRAS and EGFR was investigated by direct sequencing (DS). KRAS mutations were also assessed by mutant-enriched sequencing (ME-sequencing). DS detected KRAS mutations in 16 (19%) of 83 tumors; ME-sequencing identified all the mutations detected by DS but also mutations in minor clones of 14 additional tumors, for a total of 30 (36%) of 83. KRAS mutations assessed by DS and ME-sequencing significantly correlated with resistance to TKIs (P = .04 and P = .004, respectively) and significantly affected progression-free survival (PFS) and overall survival (OS). However, the predictive power of mutations assessed by ME-sequencing was higher than that obtained by DS (hazard ratio [HR] = 2.82, P = .0001 vs HR = 1.98, P = .04, respectively, for OS; HR = 2.52, P = .0005 vs HR = 2.21, P = .007, respectively, for PFS). Survival outcome of patients harboring KRAS mutations in minor clones, detected only by ME-sequencing, did not differ from that of patients with KRAS mutations detected by DS. Only KRAS mutations assessed by ME-sequencing remained an independent predictive factor at multivariate analysis. KRAS mutations in minor clones have an important impact on response and survival of patients with lung adenocarcinoma treated with EGFR-TKI. The use of sensitive detection methods could allow to more effectively identify treatment-resistant patients.
Figures
Figure 1
Schematic diagram showing the ME-sequencing method. See the Materials and Methods section for a detailed description. A, B, and C are the primers used for PCR amplification. The symbol (●) on the primers indicates a mismatch from the genomic normal KRAS sequence, which gives rise to a restriction site for the _Bst_NI enzyme.
Figure 2
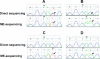
Representative examples of matched DS and ME-sequencing chromatograms for KRAS analysis of lung adenocarcinoma samples. (A and B) Tumors in which KRAS mutations (arrows) were visible with both DS and ME-sequencing. (C and D) cases in which the mutations were detectable only by ME-sequencing (arrows). The enriched procedure is so effective that only the mutated allele is evident.
Figure 3
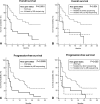
Overall (A and B) and progression-free (C and D) survival curves in lung adenocarcinoma patients according to the mutational status of KRAS assessed by ME-sequencing (A and C), by DS or only by ME-sequencing (B and D), see test for details. Curve differences are statistically significant. All P values refer to log-rank tests. Months indicate months from the beginning of treatment.
Figure 4
Overall (A) and progression-free (B) survival curves in lung adenocarcinoma patients according to the mutational status of KRAS assessed by DS. Curve differences are statistically significant. All P values refer to log-rank tests. Months indicate months from the beginning of treatment.
Figure 5
Overall (A) and progression-free (B) survival curves in lung adenocarcinoma patients according to the mutational status of EGFR. Curve differences are statistically significant. All P values refer to log-rank tests. Months indicate months from the beginning of treatment.
Figure 6
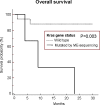
Overall curves in patients with lung adenocarcinoma harboring EGFR mutations according to the mutational status of KRAS assessed by ME-sequencing. Curve differences are statistically significant. The P value refers to log-rank test. Months indicate months from the beginning of treatment.
Similar articles
- Impact of systematic EGFR and KRAS mutation evaluation on progression-free survival and overall survival in patients with advanced non-small-cell lung cancer treated by erlotinib in a French prospective cohort (ERMETIC project--part 2).
Cadranel J, Mauguen A, Faller M, Zalcman G, Buisine MP, Westeel V, Longchampt E, Wislez M, Coudert B, Daniel C, Chetaille B, Michiels S, Blons H, Solassol J, De Fraipont F, Foucher P, Urban T, Lacroix L, Poulot V, Quoix E, Antoine M, Danton G, Morin F, Chouaid C, Pignon JP. Cadranel J, et al. J Thorac Oncol. 2012 Oct;7(10):1490-502. doi: 10.1097/JTO.0b013e318265b2b5. J Thorac Oncol. 2012. PMID: 22982650 - EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer.
van Zandwijk N, Mathy A, Boerrigter L, Ruijter H, Tielen I, de Jong D, Baas P, Burgers S, Nederlof P. van Zandwijk N, et al. Ann Oncol. 2007 Jan;18(1):99-103. doi: 10.1093/annonc/mdl323. Epub 2006 Oct 23. Ann Oncol. 2007. PMID: 17060486 - Effectiveness of erlotinib treatment in advanced KRAS mutation-negative lung adenocarcinoma patients: Results of a multicenter observational cohort study (MOTIVATE).
Sarosi V, Losonczy G, Francovszky E, Tolnay E, Torok S, Galffy G, Hegedus B, Dome B, Ostoros G. Sarosi V, et al. Lung Cancer. 2014 Oct;86(1):54-8. doi: 10.1016/j.lungcan.2014.07.011. Epub 2014 Jul 27. Lung Cancer. 2014. PMID: 25129367 - Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway.
Nguyen KS, Kobayashi S, Costa DB. Nguyen KS, et al. Clin Lung Cancer. 2009 Jul;10(4):281-9. doi: 10.3816/CLC.2009.n.039. Clin Lung Cancer. 2009. PMID: 19632948 Free PMC article. Review. - First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.
Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. Greenhalgh J, et al. Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. PMID: 27223332 Updated. Review.
Cited by
- Allele specific locked nucleic acid quantitative PCR (ASLNAqPCR): an accurate and cost-effective assay to diagnose and quantify KRAS and BRAF mutation.
Morandi L, de Biase D, Visani M, Cesari V, De Maglio G, Pizzolitto S, Pession A, Tallini G. Morandi L, et al. PLoS One. 2012;7(4):e36084. doi: 10.1371/journal.pone.0036084. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558339 Free PMC article. - Molecular heterogeneity assessment by next-generation sequencing and response to gefitinib of EGFR mutant advanced lung adenocarcinoma.
Bria E, Pilotto S, Amato E, Fassan M, Novello S, Peretti U, Vavalà T, Kinspergher S, Righi L, Santo A, Brunelli M, Corbo V, Giglioli E, Sperduti I, Milella M, Chilosi M, Scarpa A, Tortora G. Bria E, et al. Oncotarget. 2015 May 20;6(14):12783-95. doi: 10.18632/oncotarget.3727. Oncotarget. 2015. PMID: 25904052 Free PMC article. - Lung cancer in never smokers.
Yang P. Yang P. Semin Respir Crit Care Med. 2011 Feb;32(1):10-21. doi: 10.1055/s-0031-1272865. Epub 2011 Apr 15. Semin Respir Crit Care Med. 2011. PMID: 21500120 Free PMC article. Review. - [Histology-based algorithm in the molecular diagnosis of mutations of the Epidernal Growth Factor Receptor (EGFR) in non-small cell lung cancer].
Popper H, Wrba F, Gruber-Mösenbacher U, Hulla W, Pirker R, Hilbe W, Studnicka M, Mohn-Staudner A, Ploner F; und die Arbeitsgruppe Pulmopathologie der ÖGP-IAP. Popper H, et al. Wien Klin Wochenschr. 2011 May;123(9-10):316-21. doi: 10.1007/s00508-011-1573-8. Epub 2011 May 31. Wien Klin Wochenschr. 2011. PMID: 21604158 German. - KRAS mutant tumor subpopulations can subvert durable responses to personalized cancer treatments.
Parsons BL, Myers MB. Parsons BL, et al. Per Med. 2013 Mar;10(2):191-199. doi: 10.2217/pme.13.1. Per Med. 2013. PMID: 27867401 Free PMC article.
References
- Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20(Suppl 18):1–13. - PubMed
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small cell lung cancer (The IDEAL 1 Trial) J Clin Oncol. 2003;21:2237–2246. - PubMed
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. - PubMed
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous