Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome - PubMed (original) (raw)
Comparative Study
Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome
Christopher J Yuskaitis et al. Biochem Pharmacol. 2010.
Abstract
Fragile X syndrome (FXS), the most common form of inherited mental retardation and a genetic cause of autism, results from mutated fragile X mental retardation-1 (Fmr1). This study examined the effects on glycogen synthase kinase-3 (GSK3) of treatment with a metabotropic glutamate receptor (mGluR) antagonist, MPEP, and the GSK3 inhibitor, lithium, in C57Bl/6 Fmr1 knockout mice. Increased mGluR signaling may contribute to the pathology of FXS, and the mGluR5 antagonist MPEP increased inhibitory serine-phosphorylation of brain GSK3 selectively in Fmr1 knockout mice but not in wild-type mice. Inhibitory serine-phosphorylation of GSK3 was lower in Fmr1 knockout, than wild-type, mouse brain regions and was increased by acute or chronic lithium treatment, which also increased hippocampal brain-derived neurotrophic factor levels. Fmr1 knockout mice displayed alterations in open-field activity, elevated plus-maze, and passive avoidance, and these differences were ameliorated by chronic lithium treatment. These findings support the hypothesis that impaired inhibition of GSK3 contributes to the pathogenesis of FXS and support GSK3 as a potential therapeutic target.
Figures
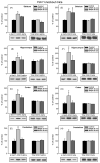
Fig. 1
Acute MPEP administration increases GSK3 serine-phosphorylation selectively in Fmr1 knockout mice. Fmr1 knockout mice were administered MPEP (30 mg/kg; ip) or vehicle 30 or 90 min prior to sacrifice. Extracts of striatum, hippocampus, cerebral cortex, and cerebellum were probed with antibodies to (A–D) phospho-Ser21-GSK3α or total GSK3α, and (E–H) phospho-Ser9-GSK3β or total GSK3β. Shown are representative immunoblots and quantitative values presented as the percent of values from untreated mice analyzed on the same gel. n = 6 mice for control and 30 min groups, and n = 3 for 90 min group; *p < 0.05; †p < 0.06 compared to untreated values.
Fig. 2
Acute MPEP administration does not change GSK3 serine-phosphorylation in wild-type mice. Wild-type mice were administered MPEP (30 mg/kg; ip) or vehicle 30 or 90 min prior to sacrifice. Extracts of striatum, hippocampus, cerebral cortex, and cerebellum were probed with antibodies to (A–D) phospho-Ser21-GSK3α or total GSK3α, and (E–H,) phospho-Ser9-GSK3β or total GSK3β. Shown are representative immunoblots and quantitative values presented as the percent of values from untreated mice analyzed on the same gel. n = 6 mice for control and 30 min groups, and n = 3 for 90 min group; *p < 0.05 compared to untreated values.
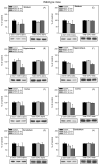
Fig. 3
GSK3 serine-phosphorylation modulated by acute lithium administration. (A) Fmr1 knockout and (B) wild-type mice were administered lithium chloride (ip; 4 mmole/kg) in PBS for 30, 60, or 180 min. Extracts of striatum, hippocampus, cerebral cortex, and cerebellum were immunoblotted for phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, total GSK3α, or total GSK3β. Shown are representative immunoblots and quantitative values presented as the percent of values from untreated mice analyzed on the same gel. n = 4 mice per group; *p < 0.05 compared to untreated values.
Fig. 3
GSK3 serine-phosphorylation modulated by acute lithium administration. (A) Fmr1 knockout and (B) wild-type mice were administered lithium chloride (ip; 4 mmole/kg) in PBS for 30, 60, or 180 min. Extracts of striatum, hippocampus, cerebral cortex, and cerebellum were immunoblotted for phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, total GSK3α, or total GSK3β. Shown are representative immunoblots and quantitative values presented as the percent of values from untreated mice analyzed on the same gel. n = 4 mice per group; *p < 0.05 compared to untreated values.
Fig. 4
Chronic lithium treatment rescues hyperactive GSK3 in Fmr1 knockout mice. Fmr1 knockout (Fragile X) and wild-type mice were treated with lithium for 3–4 weeks prior to sacrifice and compared to untreated littermates. Homogenates of the striatum, hippocampus, cerebral cortex, and cerebellum were probed with antibodies to (A–D) phospho-Ser21-GSK3α, (E–H) total GSK3α, (I–L) phospho-Ser9-GSK3β, (M-P) or total GSK3β. Immunoblots were quantified by densitometry and are presented as the percents of values from untreated wild-type mice. n = 10 mice per group; **p < 0.05 comparing untreated Fragile X and wild-type values; *p < 0.05 compared with matched sample without lithium treatment.
Fig. 4
Chronic lithium treatment rescues hyperactive GSK3 in Fmr1 knockout mice. Fmr1 knockout (Fragile X) and wild-type mice were treated with lithium for 3–4 weeks prior to sacrifice and compared to untreated littermates. Homogenates of the striatum, hippocampus, cerebral cortex, and cerebellum were probed with antibodies to (A–D) phospho-Ser21-GSK3α, (E–H) total GSK3α, (I–L) phospho-Ser9-GSK3β, (M-P) or total GSK3β. Immunoblots were quantified by densitometry and are presented as the percents of values from untreated wild-type mice. n = 10 mice per group; **p < 0.05 comparing untreated Fragile X and wild-type values; *p < 0.05 compared with matched sample without lithium treatment.
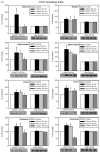
Fig. 5
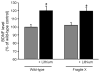
Chronic lithium treatment increases hippocampal BDNF levels. Fmr1 knockout and wild-type mice were treated with lithium for 3–4 weeks prior to sacrifice and compared to untreated littermates. BDNF levels were measured in hippocampal extracts by ELISA. Results are expressed as a percent of values in untreated wild-type controls; n = 10 mice per group; *p < 0.05 compared with matched sample without lithium treatment.
Fig. 6
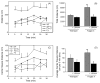
Chronic lithium treatment rescues hyperactive behavior of Fmr1 knockout mice. Fmr1 knockout (FX) and wild-type (WT) mice were treated with lithium for 3–4 weeks prior to 30 min open-field testing of activity. (A) Distance traveled was analyzed in 5 min bins. (B) Total, cumulative distance traveled during the 30 min test. (C) Center-square behavior, defined as the central zone of the open-field, was measured as distance traveled in the central area in 5 min bins. (D) Average center-square distance per 5 min bin. n = 5 mice per group. **p < 0.05 compared to untreated, wild-type values; *p < 0.05 compared with matched sample without lithium treatment.
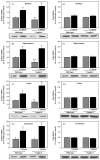
Fig. 7
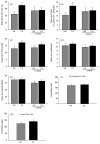
Lithium partially rescues altered elevated plus-maze behavior of Fmr1 knockout mice, and Fmr1 knockout mice do not display depressive-like behaviors. Fmr1 knockout (FX) and wild-type (WT) mice were treated with lithium for 3–4 weeks prior to testing. Behavior in the 5 min elevated plus-maze test was analyzed as (A) total time in the open arms, (B) percentage of time spent in the open arms compared with total time spent in all arms, (C) closed arm entries, (D) open arm entries, and (E) open arm explorations. (F) Immobility time was measured during the last 4 min of the 6 min tail suspension test. (G) Immobility time was measured by beam breaks in the forced swim test. n = 10 mice per group; *p < 0.05 compared to untreated, wild-type values.
Fig. 8
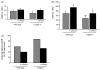
Chronic lithium treatment rescues impaired passive avoidance behavior of Fmr1 knockout mice. Fmr1 knockout and wild-type mice were treated with lithium for 3–4 weeks prior to behavioral assessment. (A) On training day, the latency to enter the dark chamber was measured during a 60 s test. Mice remaining in the light chamber for 60 s were excluded from further analysis. (B) Latency to enter the dark chamber 24 h after training was measured, with a cutoff time of 9 min. (C) Percentage of mice in each group crossing into the dark chamber within the 9 min cutoff time. n = 15 mice per group; **p < 0.05 compared to untreated, wild-type values; *p < 0.05 compared with matched sample without lithium treatment.
Similar articles
- Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome.
Yuskaitis CJ, Beurel E, Jope RS. Yuskaitis CJ, et al. Biochim Biophys Acta. 2010 Nov;1802(11):1006-12. doi: 10.1016/j.bbadis.2010.06.015. Epub 2010 Jul 1. Biochim Biophys Acta. 2010. PMID: 20600866 Free PMC article. - GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism.
Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. Mines MA, et al. PLoS One. 2010 Mar 16;5(3):e9706. doi: 10.1371/journal.pone.0009706. PLoS One. 2010. PMID: 20300527 Free PMC article. - Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice.
Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS. Franklin AV, et al. Biol Psychiatry. 2014 Feb 1;75(3):198-206. doi: 10.1016/j.biopsych.2013.08.003. Epub 2013 Sep 13. Biol Psychiatry. 2014. PMID: 24041505 Free PMC article. - BDNF in fragile X syndrome.
Castrén ML, Castrén E. Castrén ML, et al. Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review. - Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development.
Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F. Pop AS, et al. Psychopharmacology (Berl). 2014 Mar;231(6):1217-26. doi: 10.1007/s00213-013-3330-3. Psychopharmacology (Berl). 2014. PMID: 24232444 Review.
Cited by
- Potential therapeutic interventions for fragile X syndrome.
Levenga J, de Vrij FM, Oostra BA, Willemsen R. Levenga J, et al. Trends Mol Med. 2010 Nov;16(11):516-27. doi: 10.1016/j.molmed.2010.08.005. Epub 2010 Sep 21. Trends Mol Med. 2010. PMID: 20864408 Free PMC article. Review. - Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome.
Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Jung KM, et al. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. Nat Commun. 2012. PMID: 23011134 Free PMC article. - Pharmacological rescue of cortical synaptic and network potentiation in a mouse model for fragile X syndrome.
Chen T, Lu JS, Song Q, Liu MG, Koga K, Descalzi G, Li YQ, Zhuo M. Chen T, et al. Neuropsychopharmacology. 2014 Jul;39(8):1955-67. doi: 10.1038/npp.2014.44. Epub 2014 Feb 20. Neuropsychopharmacology. 2014. PMID: 24553731 Free PMC article. - Drug discovery for autism spectrum disorder: challenges and opportunities.
Ghosh A, Michalon A, Lindemann L, Fontoura P, Santarelli L. Ghosh A, et al. Nat Rev Drug Discov. 2013 Oct;12(10):777-90. doi: 10.1038/nrd4102. Nat Rev Drug Discov. 2013. PMID: 24080699 Review. - Learning and behavioral deficits associated with the absence of the fragile X mental retardation protein: what a fly and mouse model can teach us.
Santos AR, Kanellopoulos AK, Bagni C. Santos AR, et al. Learn Mem. 2014 Sep 16;21(10):543-55. doi: 10.1101/lm.035956.114. Print 2014 Oct. Learn Mem. 2014. PMID: 25227249 Free PMC article. Review.
References
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–22. - PubMed
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev. 2006;16:270–5. - PubMed
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–6. - PubMed
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DA024761/DA/NIDA NIH HHS/United States
- MH57014/MH/NIMH NIH HHS/United States
- R56 MH038752/MH/NIMH NIH HHS/United States
- MH38752/MH/NIMH NIH HHS/United States
- R01 MH038752/MH/NIMH NIH HHS/United States
- R01 MH057014/MH/NIMH NIH HHS/United States
- R01 MH038752-25/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases